filmov
tv
Numerical solution of the 3D Schrodinger equation for the hydrogen atom

Показать описание
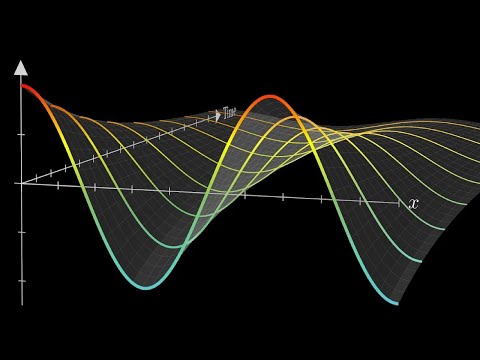
Simulation of the time evolution of the wave equation for an electron in a hydrogen atom:
ih∂ρ/∂t=-h*h/(2m) Δρ- kρ/r
Where i is the imaginary number, ρ is the dependent variable (the square of which gives the probability density), h a constant, m the particle mass, r the radial coordinate, t time and k a constant depending on the charge of the particle. The initial value of ρ at t = 0 is 0.5 + 0.5i, i.e: ρ(r,θ,ϕ,0) = 0.5 + 0.5i. The graphs are the solutions as a function of the radial coordinate. The blue line gives the real part of the solution and the orange line the imaginary part.
The code can be found at:
ih∂ρ/∂t=-h*h/(2m) Δρ- kρ/r
Where i is the imaginary number, ρ is the dependent variable (the square of which gives the probability density), h a constant, m the particle mass, r the radial coordinate, t time and k a constant depending on the charge of the particle. The initial value of ρ at t = 0 is 0.5 + 0.5i, i.e: ρ(r,θ,ϕ,0) = 0.5 + 0.5i. The graphs are the solutions as a function of the radial coordinate. The blue line gives the real part of the solution and the orange line the imaginary part.
The code can be found at:
 0:00:21
0:00:21
 0:00:46
0:00:46
 0:02:17
0:02:17
 0:14:13
0:14:13
 0:00:48
0:00:48
 0:33:23
0:33:23
 0:06:25
0:06:25
 0:00:11
0:00:11
 0:03:40
0:03:40
 0:04:27
0:04:27
 0:17:39
0:17:39
 1:20:38
1:20:38
 0:02:13
0:02:13
 0:00:16
0:00:16
 0:07:01
0:07:01
 0:03:11
0:03:11
 0:00:34
0:00:34
 0:00:16
0:00:16
 0:00:20
0:00:20
 0:00:27
0:00:27
 0:08:35
0:08:35
 0:15:22
0:15:22
 0:44:56
0:44:56
 0:35:02
0:35:02