filmov
tv
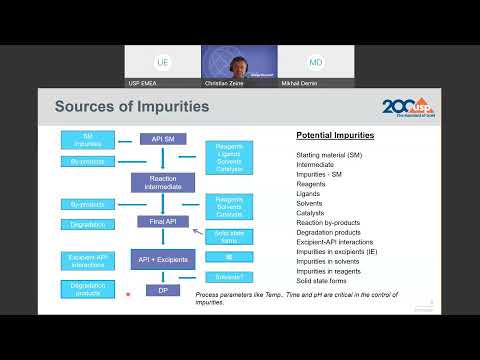
Related Impurities Assessment Considerations for APIs in the Generic Complex Peptide Products

Показать описание
Manivannan Ethirajan from the Office of New Drug Products (ONDP) in the Office of Pharmaceutical Quality outlines the specification considerations for peptide-related impurities in Active Pharmaceutical Ingredients (APIs) of the generic complex peptide drugs as the ICH guideline (Q3A) does not apply to these generic peptide drug substances. Discussion includes current thinking and recommendations.
_______________________________
FDA CDER’s Small Business and Industry Assistance (SBIA) educates and provides assistance in understanding the regulatory aspects of human drug products & clinical research.
Phone - (301) 796-6707 I (866) 405-5367
_______________________________
FDA CDER’s Small Business and Industry Assistance (SBIA) educates and provides assistance in understanding the regulatory aspects of human drug products & clinical research.
Phone - (301) 796-6707 I (866) 405-5367
 0:20:18
0:20:18
 0:03:57
0:03:57
 0:26:07
0:26:07
 0:08:41
0:08:41
 0:18:11
0:18:11
 0:22:14
0:22:14
 0:21:51
0:21:51
 0:34:21
0:34:21
 0:20:38
0:20:38
 0:55:13
0:55:13
 0:15:42
0:15:42
 0:03:40
0:03:40
 0:34:21
0:34:21
 0:23:16
0:23:16
 0:39:13
0:39:13
 0:12:22
0:12:22
 1:01:10
1:01:10
 0:40:39
0:40:39
 0:15:03
0:15:03
 0:07:19
0:07:19
 0:23:08
0:23:08
 0:47:29
0:47:29
 1:22:14
1:22:14
 0:00:41
0:00:41