filmov
tv
Exploring the Science of Enzymes: Activation Energy Demystified

Показать описание
📢 Receive Comprehensive Mathematics Practice Papers Weekly for FREE 😊
If enzymes did not exist, reactions in our body would rely on being processed spontaneously and these reactions do not occur fast enough for our body to cope with that alone. For example, the waste products would build up in our body and our digestive system would not be as efficient. Enzyme catalysts are able to speed up (or slow down) reactions without a change in temperature.
This is extremely important in cells since heat damages living tissue. For a chemical reaction to begin, activation energy is necessary. The presence of enzymes means our bodies require less energy than if reactions were not catalysed.
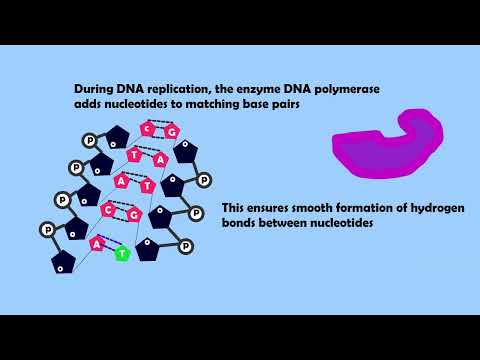
This is called activation energy. This energy is needed to break down chemical bonds allowing the reaction to occur. Enzymes themselves are not altered or used up in the process of this reaction. When an enzyme binds to a substrate, it lowers the energy needed by the substrate molecules to react to form products. This increases the chance of the reaction occurring and therefore increases the rate of reaction.
The role of an enzyme is to lower the activation energy needed to start a reaction, so that the reaction can proceed quickly, without a change in temperature. It is important to remember that an enzyme does not provide activation energy—it reduces the amount of activation energy needed (by bringing specific molecules together, rather than relying on them colliding randomly).
If enzymes did not exist, reactions in our body would rely on being processed spontaneously and these reactions do not occur fast enough for our body to cope with that alone. For example, the waste products would build up in our body and our digestive system would not be as efficient. Enzyme catalysts are able to speed up (or slow down) reactions without a change in temperature.
This is extremely important in cells since heat damages living tissue. For a chemical reaction to begin, activation energy is necessary. The presence of enzymes means our bodies require less energy than if reactions were not catalysed.
This is called activation energy. This energy is needed to break down chemical bonds allowing the reaction to occur. Enzymes themselves are not altered or used up in the process of this reaction. When an enzyme binds to a substrate, it lowers the energy needed by the substrate molecules to react to form products. This increases the chance of the reaction occurring and therefore increases the rate of reaction.
The role of an enzyme is to lower the activation energy needed to start a reaction, so that the reaction can proceed quickly, without a change in temperature. It is important to remember that an enzyme does not provide activation energy—it reduces the amount of activation energy needed (by bringing specific molecules together, rather than relying on them colliding randomly).
Комментарии
 0:02:52
0:02:52
 0:03:56
0:03:56
 0:05:47
0:05:47
 0:00:59
0:00:59
 0:02:37
0:02:37
 0:00:45
0:00:45
 0:02:43
0:02:43
 0:00:51
0:00:51
 0:43:52
0:43:52
 0:00:41
0:00:41
 0:01:36
0:01:36
 0:02:07
0:02:07
 0:00:18
0:00:18
 0:00:19
0:00:19
 0:00:07
0:00:07
 0:00:10
0:00:10
 0:09:55
0:09:55
 0:00:54
0:00:54
 0:02:07
0:02:07
 0:00:15
0:00:15
 0:00:49
0:00:49
 0:01:29
0:01:29
 0:00:21
0:00:21
 0:00:55
0:00:55