filmov
tv
Distinguish Between Sigma (𝛔) & Pi (𝝅) Molecular Orbitals.
Показать описание
𝛔 (sigma) Molecular Orbitals are formed when the atomic orbitals overlap along the line joining the two nuclei (head-on). These are cylindrically symmetrical along the inter nuclear axis.
1. The lobes of the Atomic Orbitals are in line with the internuclear axis.
2. Formed by head on collision of Atomic Orbitals
3. The electron density along the internuclear axis is maximum
4. Bonding M.Os are gerade and anti-bonding M.Os are ungerade.
5. Forms stronger bond.
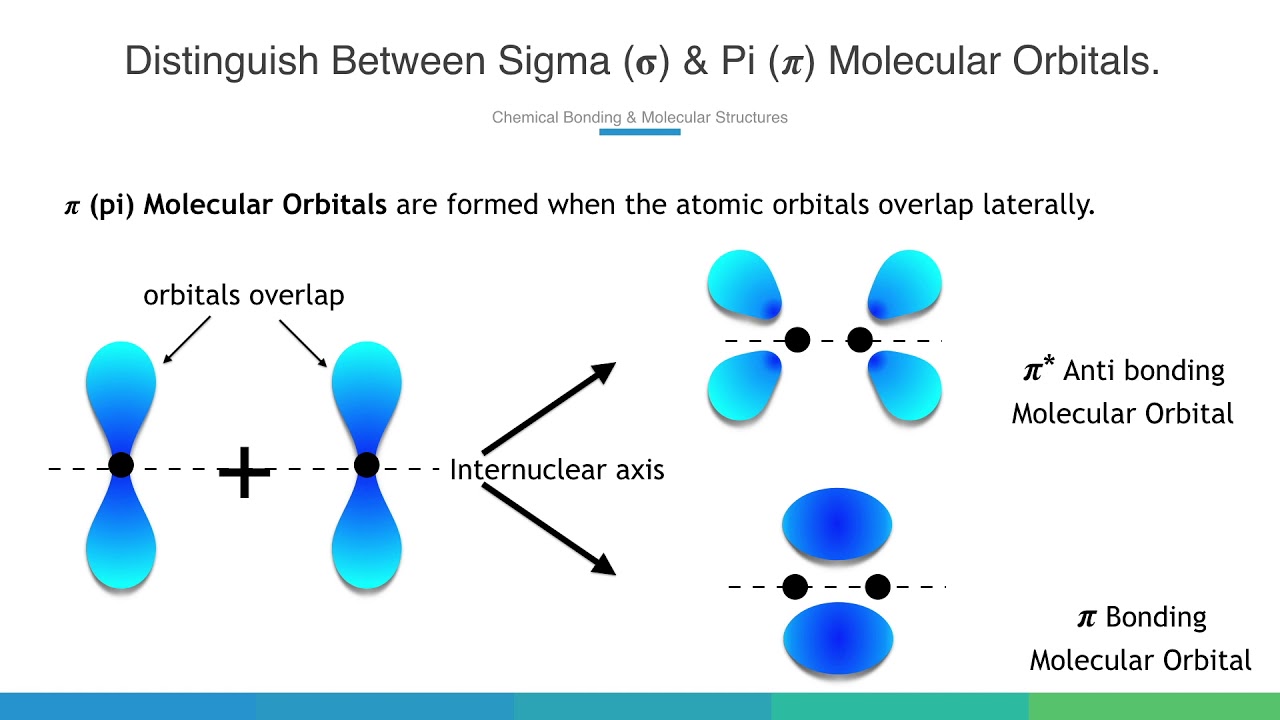
𝝅 (pi) Molecular Orbitals are formed when the atomic orbitals overlap laterally.
1. The lobes of Atomic Orbitals are perpendicular to the internuclear axis.
2. Formed by lateral overlap of Atomic Orbitals
3. The electron density along the internuclear axis is zero
4. Bonding M.Os are ungerade and antibonding M.Os are gerade.
5. Forms comparatively weaker bond.
Other Subjects:
1. The lobes of the Atomic Orbitals are in line with the internuclear axis.
2. Formed by head on collision of Atomic Orbitals
3. The electron density along the internuclear axis is maximum
4. Bonding M.Os are gerade and anti-bonding M.Os are ungerade.
5. Forms stronger bond.
𝝅 (pi) Molecular Orbitals are formed when the atomic orbitals overlap laterally.
1. The lobes of Atomic Orbitals are perpendicular to the internuclear axis.
2. Formed by lateral overlap of Atomic Orbitals
3. The electron density along the internuclear axis is zero
4. Bonding M.Os are ungerade and antibonding M.Os are gerade.
5. Forms comparatively weaker bond.
Other Subjects:
Комментарии