filmov
tv
Topic 1 - Clinical Trial eRecruitment + Q&A | #2 Webinar | A Trust-Centric Healthcare Journey II

Показать описание
#Blockchain #ClinicalTrial #eRecruitment #Pharmaledger #PLwebinar2021
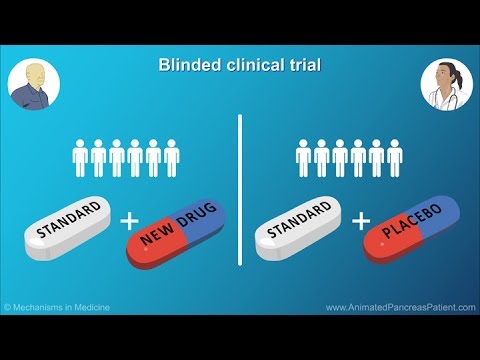
Watch the recording of PharmaLedger’s Clinical Trial eRecruitment use case presented during our second Open Webinar: A Trust-Centric Healthcare Journey Part II. This is an overview of PharmaLedger’s blockchain-enabled clinical trial and health data use cases, which took place on February 24th and March 3rd, 2021.
PharmaLedger partners joined together to present a number of ongoing challenges faced within pharmaceutical clinical trials and health data, along with the added value of using blockchain for all stakeholders involved in each use case.
This video includes Topic 1 - eRecruitment, presented by:
Despina Daliani (Onorach) and Ken Nessel (Pfizer)
Time Stamps | Presentation
Introduction to Clinical Trials (0:55)
Current Situation: Clinical Trial Recruitment (2:30)
Challenges (4:49)
Future Vision (5:58)
The Patient-Empowerment Experience (8:05)
Why Blockchain for Clinical Trial Recruitment? (9:42)
Value Proposition to Stakeholders (10:30)
Roadmap (11:30)
Poll Results (12:47)
Q&A (14:07)
- Have you analysed Patient Matching Utilities relation to GDPR? Who would be the data controller? (14:15)
- How would you communicate that this utility is safe? the communication would be worldwide and not specifically targeted-would that be cost expensive? (16:13)
- Are there considerations about monetary incentives for patients providing data for clinical trials? (17:30)
- How would you guarantee that no excess information is given (or asked for) to a company that does not need these data for trials? (18:49)
- Will there be impact on inclusiveness if patients need to access an app to be included in trials if they don't have a smartphone? Any solution for that? (21:02)
- Recruitment- how would governance work for such a matching tool? (23:05)
- Blockchain has been used without specificity. Which 'blockchain' platform? And how do you intend to scale the voluminous data processing and use? (24:38)
- Who/which suppliers would make these blockchains a reality? This is costly work and there are no off-the-shelf solutions. (27:10)
- Can you please describe the process for achieving agreement on the data governance by all consortiums for all/any of these studies? (28:15)
- How to join PharmaLedger ? We have developed an scalable use case that fits 100%. (30:06)
- When could patients expect solutions to be available? (31:58)
- Given that there are other similar solutions in development, how will you build the critical mass to ensure yours is successful? (33:28)
- Will Big Pharma own these solutions? (34:30)
- How many ledgers and nodes do you have on a possible chain code? (35:17)
--------
You can watch the other separate use cases or see the full recording on PharmaLedger's Youtube channel. This is part of a series of webinars aimed at fostering greater alignment and a common understanding of PharmaLedger’s activities, approaches and progress among its members and the general public.
Find us @pharmaledger | Twitter + LinkedIn + Facebook
This work has received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking grant n° 853992
Watch the recording of PharmaLedger’s Clinical Trial eRecruitment use case presented during our second Open Webinar: A Trust-Centric Healthcare Journey Part II. This is an overview of PharmaLedger’s blockchain-enabled clinical trial and health data use cases, which took place on February 24th and March 3rd, 2021.
PharmaLedger partners joined together to present a number of ongoing challenges faced within pharmaceutical clinical trials and health data, along with the added value of using blockchain for all stakeholders involved in each use case.
This video includes Topic 1 - eRecruitment, presented by:
Despina Daliani (Onorach) and Ken Nessel (Pfizer)
Time Stamps | Presentation
Introduction to Clinical Trials (0:55)
Current Situation: Clinical Trial Recruitment (2:30)
Challenges (4:49)
Future Vision (5:58)
The Patient-Empowerment Experience (8:05)
Why Blockchain for Clinical Trial Recruitment? (9:42)
Value Proposition to Stakeholders (10:30)
Roadmap (11:30)
Poll Results (12:47)
Q&A (14:07)
- Have you analysed Patient Matching Utilities relation to GDPR? Who would be the data controller? (14:15)
- How would you communicate that this utility is safe? the communication would be worldwide and not specifically targeted-would that be cost expensive? (16:13)
- Are there considerations about monetary incentives for patients providing data for clinical trials? (17:30)
- How would you guarantee that no excess information is given (or asked for) to a company that does not need these data for trials? (18:49)
- Will there be impact on inclusiveness if patients need to access an app to be included in trials if they don't have a smartphone? Any solution for that? (21:02)
- Recruitment- how would governance work for such a matching tool? (23:05)
- Blockchain has been used without specificity. Which 'blockchain' platform? And how do you intend to scale the voluminous data processing and use? (24:38)
- Who/which suppliers would make these blockchains a reality? This is costly work and there are no off-the-shelf solutions. (27:10)
- Can you please describe the process for achieving agreement on the data governance by all consortiums for all/any of these studies? (28:15)
- How to join PharmaLedger ? We have developed an scalable use case that fits 100%. (30:06)
- When could patients expect solutions to be available? (31:58)
- Given that there are other similar solutions in development, how will you build the critical mass to ensure yours is successful? (33:28)
- Will Big Pharma own these solutions? (34:30)
- How many ledgers and nodes do you have on a possible chain code? (35:17)
--------
You can watch the other separate use cases or see the full recording on PharmaLedger's Youtube channel. This is part of a series of webinars aimed at fostering greater alignment and a common understanding of PharmaLedger’s activities, approaches and progress among its members and the general public.
Find us @pharmaledger | Twitter + LinkedIn + Facebook
This work has received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking grant n° 853992
 0:06:42
0:06:42
 0:38:03
0:38:03
 0:03:54
0:03:54
 0:28:54
0:28:54
 0:00:14
0:00:14
 0:00:44
0:00:44
 0:17:55
0:17:55
 0:04:34
0:04:34
 0:05:24
0:05:24
 0:27:25
0:27:25
 0:49:28
0:49:28
 0:01:55
0:01:55
 0:00:58
0:00:58
 0:19:42
0:19:42
 0:06:47
0:06:47
 0:36:10
0:36:10
 0:16:00
0:16:00
 0:01:49
0:01:49
 0:12:54
0:12:54
 0:06:59
0:06:59
 0:15:28
0:15:28
 0:06:24
0:06:24
 0:52:23
0:52:23
 0:21:43
0:21:43