filmov
tv
ADA Guidelines: How Do They Come About?

Показать описание
The American Diabetes Association (ADA) 2021 guidelines were just published, and I want to take a minute to discuss how the guidelines come about. [In the next two videos], I will talk about what I think is most important in the new guidelines.
The guidelines are created by the Professional Practice Committee of the American Diabetes Association. This is a committee of 16 individuals chosen from a variety of areas in terms of their knowledge about diabetes. Every year, our task is to go through and update the former set of guidelines, and then create the next year's guidelines.
The term on the committee is generally about 2 years so that the committee does not stagnate. This is not a committee of the same people year in and year out; it's a dynamic group of people who challenge each other and really try to make good guidelines. Of those 16 people, one is designated as the chairperson, and that person works very closely with the chief medical officer (CMO) of the ADA, who is involved in guideline writing as well.
The Process
For each of the 15 or so sections of the guidelines, a person is designated as the section head. For example, I am the section head for the device portion of the guidelines. My job is to write an evidence table. I look at all of the evidence and publications that have come out in the past year and fill in the table with what is new, and then I use the new evidence to create updates to the guidelines. I do that along with two or three other people from the committee who have particular interest or knowledge about devices.
Then, at a meeting held at the end of the summer, every section [head] gets to present all of their data to the group as a whole, and we all go through everything and agree on what the new guidelines are going to be. After the chairperson for the group and the ADA CMO also agree on [the guidelines], they are presented to the ADA board of directors for their final approval. Then you get the guidelines.
It's a very rigorous process, and it is pretty formal. We [carefully] go through the steps every year to try to make the best document possible, and we are very careful to grade the evidence. We give you a grade that tells you how good we think the evidence is that is supporting our guidelines. There is level A evidence (the highest level of evidence), level B, and level C. There is also level E, which, as a clinician, is important to me because this is what we are doing clinically. This is guidance from clinicians that we are adding because we may not have data on all the things that we do. There are some things that really make clinical sense that need to be included as we guide people in the management of diabetes.
I hope you like our 2021 guidelines. We do our best. Most of all, I want to make sure that we help our patients with diabetes do better and be more able to use the tools and treatments we now have available. Thank you.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California (USC) Keck School of Medicine and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
The guidelines are created by the Professional Practice Committee of the American Diabetes Association. This is a committee of 16 individuals chosen from a variety of areas in terms of their knowledge about diabetes. Every year, our task is to go through and update the former set of guidelines, and then create the next year's guidelines.
The term on the committee is generally about 2 years so that the committee does not stagnate. This is not a committee of the same people year in and year out; it's a dynamic group of people who challenge each other and really try to make good guidelines. Of those 16 people, one is designated as the chairperson, and that person works very closely with the chief medical officer (CMO) of the ADA, who is involved in guideline writing as well.
The Process
For each of the 15 or so sections of the guidelines, a person is designated as the section head. For example, I am the section head for the device portion of the guidelines. My job is to write an evidence table. I look at all of the evidence and publications that have come out in the past year and fill in the table with what is new, and then I use the new evidence to create updates to the guidelines. I do that along with two or three other people from the committee who have particular interest or knowledge about devices.
Then, at a meeting held at the end of the summer, every section [head] gets to present all of their data to the group as a whole, and we all go through everything and agree on what the new guidelines are going to be. After the chairperson for the group and the ADA CMO also agree on [the guidelines], they are presented to the ADA board of directors for their final approval. Then you get the guidelines.
It's a very rigorous process, and it is pretty formal. We [carefully] go through the steps every year to try to make the best document possible, and we are very careful to grade the evidence. We give you a grade that tells you how good we think the evidence is that is supporting our guidelines. There is level A evidence (the highest level of evidence), level B, and level C. There is also level E, which, as a clinician, is important to me because this is what we are doing clinically. This is guidance from clinicians that we are adding because we may not have data on all the things that we do. There are some things that really make clinical sense that need to be included as we guide people in the management of diabetes.
I hope you like our 2021 guidelines. We do our best. Most of all, I want to make sure that we help our patients with diabetes do better and be more able to use the tools and treatments we now have available. Thank you.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California (USC) Keck School of Medicine and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
 0:03:35
0:03:35
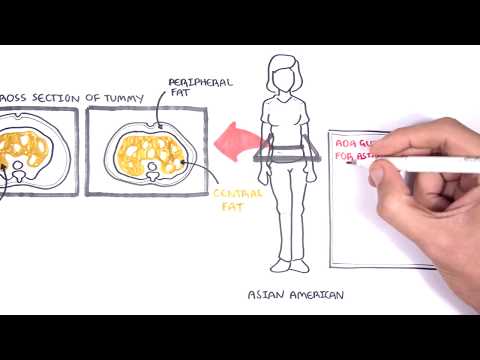 0:06:15
0:06:15
 0:06:34
0:06:34
 0:16:13
0:16:13
 0:12:18
0:12:18
 0:47:55
0:47:55
 0:09:14
0:09:14
 0:15:37
0:15:37
 0:08:15
0:08:15
 0:59:35
0:59:35
 0:13:26
0:13:26
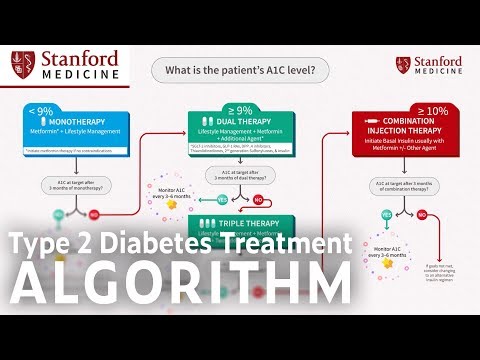 0:05:22
0:05:22
 0:16:09
0:16:09
 0:59:43
0:59:43
 0:08:44
0:08:44
 0:19:20
0:19:20
 0:08:36
0:08:36
 0:35:27
0:35:27
 0:50:21
0:50:21
 0:01:13
0:01:13
 0:05:18
0:05:18
 0:41:36
0:41:36
 0:35:10
0:35:10
 0:03:27
0:03:27