filmov
tv
Quick Revision - Clock reactions

Показать описание
Essentials of how clock reactions can be used to measure the initial rate of a reaction followed by an exam question
Quick Revision - Clock reactions
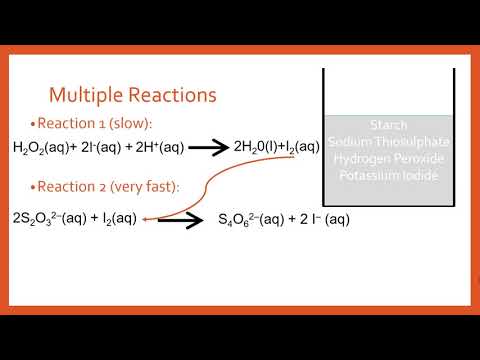
The iodine clock reaction
Iodine Clock Reaction Explanation - Mechanism and Colour Change Explained
Iodine Clock Experiment (Clock Reactions A-Level IB Chemistry)
Fastest Time Recorded for an Iodine Clock Reaction #shorts
Chemistry experiment 28 - Iodine clock reaction
Iodine Clock Reaction, Chemical Reaction
Iodine Clock Reaction
Awesome iodine clock reaction in slow motion
Iodine Clock Reaction
my favourite revision method (revision clocks)
Iodine Clock Reaction #shorts
Iodine clock reaction year 13 A-Level Chemistry
Kinetics of the Iodine Clock Reaction | Intro & Theory
IODINE CLOCK!!!
Iodine Clock Reaction
Iodine Clock Reaction
Clock reaction
Iodine Clock - Measuring the rate of a reaction by initial rate
Iodine clock reaction 😱💥
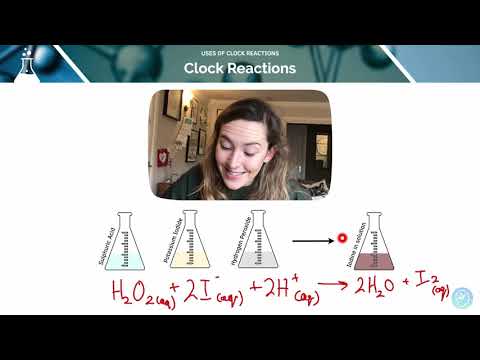
Uses of Clock Reactions | A-Level Chemistry | AQA, OCR, Edexcel
Iodine Clock Reaction
Hydrogen peroxide/Potassium iodide'Clock' Reaction 🔥
Iodine clock reaction
Комментарии
 0:02:59
0:02:59
 0:00:17
0:00:17
 0:03:36
0:03:36
 0:12:25
0:12:25
 0:00:24
0:00:24
 0:02:26
0:02:26
 0:00:10
0:00:10
 0:00:21
0:00:21
 0:01:28
0:01:28
 0:00:07
0:00:07
 0:07:20
0:07:20
 0:00:44
0:00:44
 0:03:47
0:03:47
 0:10:18
0:10:18
 0:00:05
0:00:05
 0:17:52
0:17:52
 0:00:54
0:00:54
 0:01:28
0:01:28
 0:07:05
0:07:05
 0:00:58
0:00:58
 0:04:10
0:04:10
 0:00:37
0:00:37
 0:00:12
0:00:12
 0:01:15
0:01:15