filmov
tv
OCR AS Chemistry Unit F321, Module 2 - Shells, sub shells and orbitals
Показать описание
This is an OCR AS Chemistry Unit 1, Module 2 Shells, subshells and orbitals video.
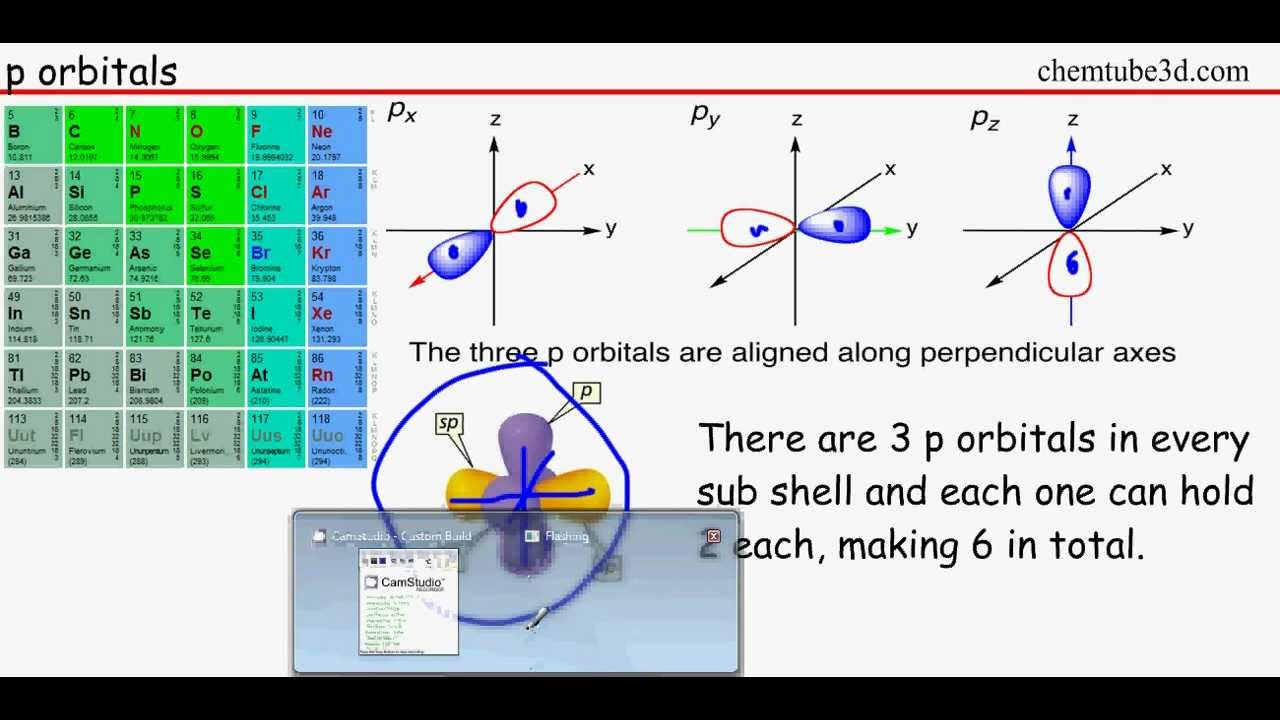
1.2.2 - Shells and orbitals
1.2.4 - Electrons and the periodic table
Sorry about the blunder in the middle - I can get distracted at times.
1.2.2 - Shells and orbitals
1.2.4 - Electrons and the periodic table
Sorry about the blunder in the middle - I can get distracted at times.
OCR AS Chemistry Unit F321, Module 2 - Shells, sub shells and orbitals
OCR AS Level Chemistry Unit F321 - Module 1 - Atomic Structure
OCR Chemistry Unit F321 Module 2 - Shapes of molecules and Ions
OCR Unit 1 F321 June 2013 Past paper work through
OCR AS Chemistry Unit 1.1.1-1.1.2 Basic Atomic Structure
F321 Atoms, Bonds and Groups May 2015 Q2d - 4a from www.ChemistryTuition.Net
OCR AS Chemistry Unit 1, Module 2 - Ionic bonding
OCR A 2.1.1 Atomic Structure and Isotopes REVISION
F321 Atoms, Bonds and Groups May 2015 Q1-2c from www.ChemistryTuition.Net
OCR AS Chemistry - Working out the Avogadro Constant from given data
OCR AS level Chemistry Unit 1 Module 1 - Atomic Mass
OCR AS Chemistry Unit 2 Module 3 - Determining enthalpy changes from surroundings
OCR AS Chemistry - Moles and Concentration Calculations
OCR AS Chemistry - Moles Calculations (using n,M and m)
OCR A 2.2.1 Electron Structure REVISION
F321 Unit 1 Titrations
OCR A 2.2.2 Bonding and structure REVISION
F321 Feb formal test
OCR AS Chemistry - Volume Calculations from masses
F321 May 14 Q1
OCR Chemistry AS Level Unit 1, Module 2- Covalent Bonding
OCR AS Chemistry - Moles and Concentration
OCR AS Chemistry - Combining moles Calculations
OCR Chemistry Unit 2 - Enthalpy changes
Комментарии
 0:12:56
0:12:56
 0:12:59
0:12:59
 0:23:18
0:23:18
 1:03:18
1:03:18
 0:10:38
0:10:38
 0:14:44
0:14:44
 0:07:41
0:07:41
 0:13:58
0:13:58
 0:11:56
0:11:56
 0:06:21
0:06:21
 0:06:08
0:06:08
 0:15:15
0:15:15
 0:06:29
0:06:29
 0:18:18
0:18:18
 0:11:58
0:11:58
 0:20:45
0:20:45
 0:25:33
0:25:33
 0:45:59
0:45:59
 0:01:10
0:01:10
 0:06:48
0:06:48
 0:07:07
0:07:07
 0:06:46
0:06:46
 0:11:36
0:11:36
 0:11:11
0:11:11