filmov
tv
Periodic Trends | Ionization Energy.
Показать описание
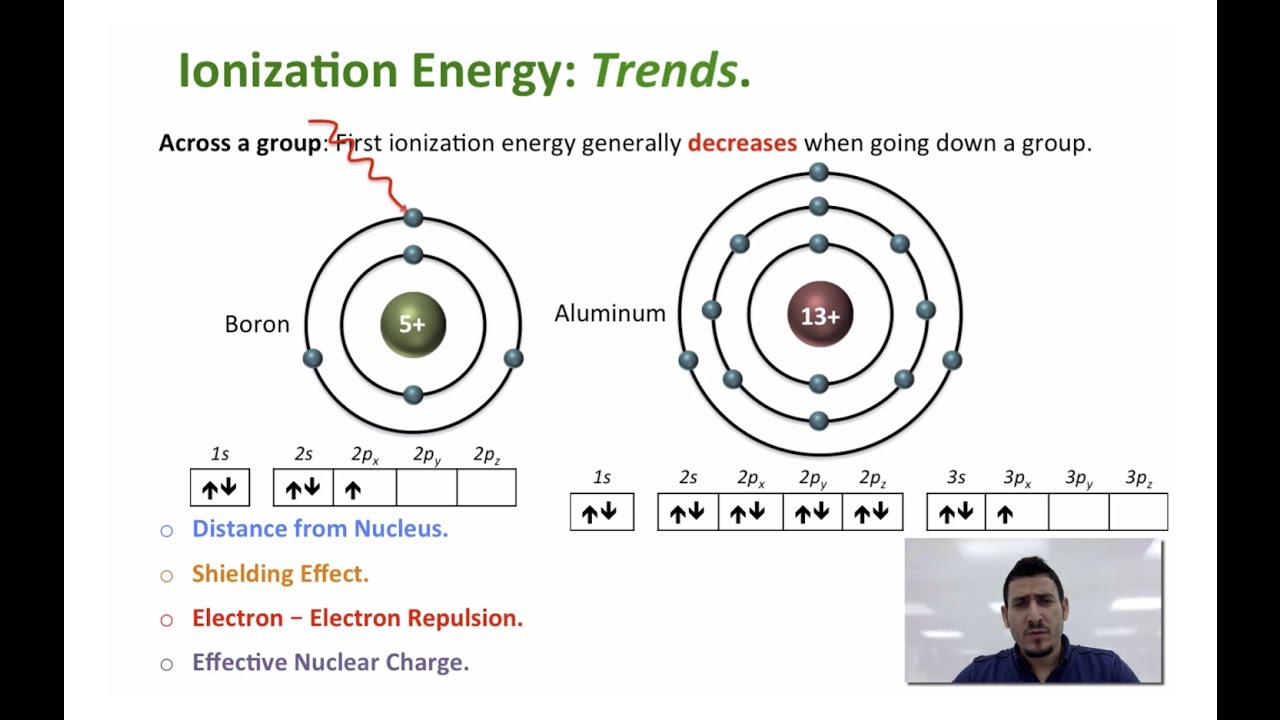
This video is about the Periodic Trends in Atomic Structure, and discusses in details the ionization energy.
In this video I discuss the trend in ionization energy:
- across a period,
- across a group.
and the exceptions to the trend:
- between Beryllium and Boron,
- between Nitrogen and Oxygen.
Moreover, the multiple ionization energies are also explained.
You can also watch: ELECTRON AFFINITY.
Students studying Chemistry at different levels could highly benefit from this video.
In this video I discuss the trend in ionization energy:
- across a period,
- across a group.
and the exceptions to the trend:
- between Beryllium and Boron,
- between Nitrogen and Oxygen.
Moreover, the multiple ionization energies are also explained.
You can also watch: ELECTRON AFFINITY.
Students studying Chemistry at different levels could highly benefit from this video.
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Ionization energy trends | Periodic table | Chemistry | Khan Academy
Periodic Trends: Ionization Energy Explained With Exceptions | Study Chemistry With Us
Ionization Energy | Periodic Trends
Ionization Energy - Basic Introduction
Periodic Trends - Atomic Radius, Electronegativity, Ionization Energy - Chemistry Series
Ionization energy trends | Atomic models and periodicity | High school chemistry | Khan Academy
7.2 Ionization Energy | Periodic Trends | General Chemistry
CHEMISTRY 101: Trends in Ionization Energies
Periodic Trends | Ionization Energy.
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE
Periodic Trends - The Trend of Ionization Energy- General Chemistry Resources
Trends in the Periodic Table
Periodic Trends: Ionization Energy - AP Chem Unit 1, Topic 7a
Periodic Trends – Ionization Energy
Periodic Table Trends: Electronegativity + Size 📈
Periodic Trends Ionization Energy
Ionization energy: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
S3.1.3 Trends in ionisation energy
Trends in Ionisation Energies | AS-Level Chemistry
Class 12 |Ch#13 |Lec #2 |Ionization energy, Trends in ionic radius#2ndyearchemistry
Ionization Energy - Periodic Trends
41: Periodic trends: Ionization energy
Periodic Trends of the Periodic Table
Комментарии
 0:07:53
0:07:53
 0:10:01
0:10:01
 0:16:42
0:16:42
 0:10:59
0:10:59
 0:32:48
0:32:48
 0:18:06
0:18:06
 0:04:32
0:04:32
 0:15:02
0:15:02
 0:03:23
0:03:23
 0:09:10
0:09:10
 0:24:55
0:24:55
 0:00:46
0:00:46
 0:09:49
0:09:49
 0:08:27
0:08:27
 0:08:30
0:08:30
 0:00:30
0:00:30
 0:07:24
0:07:24
 0:10:03
0:10:03
 0:03:08
0:03:08
 0:15:16
0:15:16
 0:21:51
0:21:51
 0:09:41
0:09:41
 0:08:40
0:08:40
 0:12:34
0:12:34