filmov
tv
Atomic Combinations grade 11 :Lewis diagram #3

Показать описание
In this lesson we are introduced to Grade 11 Lewis diagrams in atomic combinations.
Atomic Combinations grade 11 :Lewis diagram # 1
Atomic Combinations grade 11 :Lewis diagram #2
Atomic Combinations grade 11 :Lewis diagram #3
Atomic Combination Grade 11 Physical Sciences
Atomic Combinations grade 11: Polar vs Non polar
Atomic Combinations Grade 11 Exam Questions
Atomic Combinations grade 11: Electronegativity and net dipole moments #1
Grade 11 Lewis Diagrams Chemical Bonding
Atomic Combinations grade 11 Exam Questions
3. Gr 11 Physical Science - Atomic Combinations - Lewis Diagrams (Practice)
Grade 11 Atomic Combinations Chemistry Exam Questions
2. Gr 11 - Physical Science - Atomic Combinations - Lewis Diagrams (Theory)
1. Gr 11 Physical Science - Atomic Combinations - Chemical Bonds (Theory)
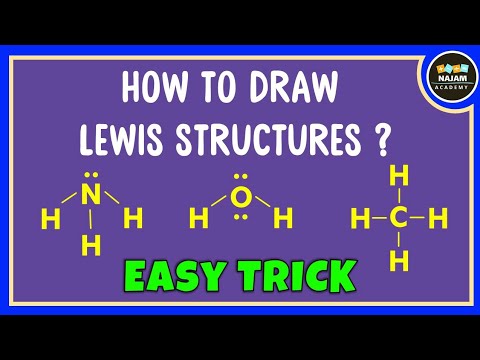
How to draw Lewis structure of any compound? Easy Trick
Atomic Combinations grade 11: Electronegativity and net dipole moments #2
Grade 11 Chemistry EXAM QUESTIONS Atomic Combinations, Polarity, Bonding part 1
Grade 11 Chemistry Atomic Combinations EXAM QUESTIONS part 2
5. Gr 11 Physical Science - Atomic Combinations - Dative Covalent Bonds (Practice)
ATOMIC COMBINATIONS GRADE 11
Physical Sciences Gr11 Matter And Materials Atomic Combinations 6 1
Chemical Bonding - CB 01
Intermolecular Forces grade 11: Different types
Intermolecular Forces grade 11: VSEPR # 1
6. Gr 11 Physical Science - Atomic Combinations - Molecular Shapes (Theory)
Комментарии
 0:06:31
0:06:31
 0:05:19
0:05:19
 0:03:20
0:03:20
 0:36:33
0:36:33
 0:06:42
0:06:42
 0:26:10
0:26:10
 0:05:04
0:05:04
 0:17:45
0:17:45
 0:29:23
0:29:23
 0:10:41
0:10:41
 0:29:47
0:29:47
 0:06:32
0:06:32
 0:06:02
0:06:02
 0:06:19
0:06:19
 0:09:13
0:09:13
 0:11:42
0:11:42
 0:20:37
0:20:37
 0:03:20
0:03:20
 0:17:08
0:17:08
 0:50:33
0:50:33
 0:22:15
0:22:15
 0:11:41
0:11:41
 0:06:16
0:06:16
 0:07:01
0:07:01