filmov
tv
Williamson Ether Synthesis Reaction Mechanism
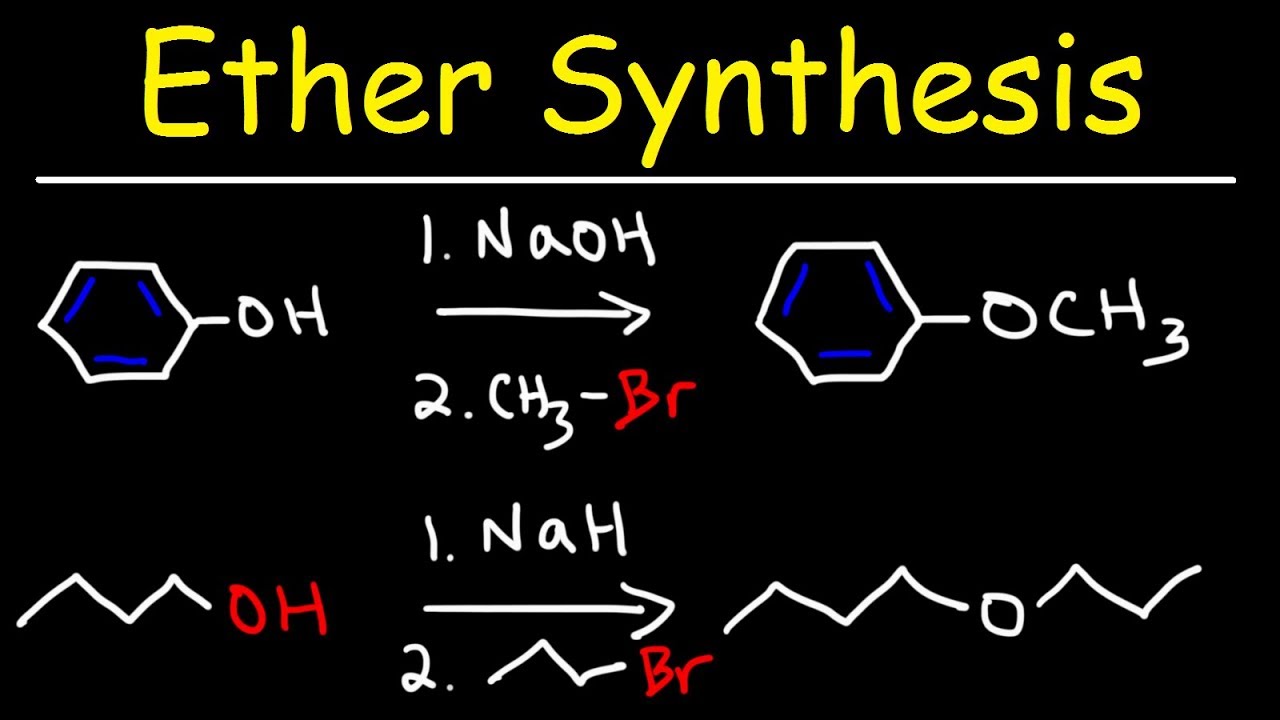
Показать описание
This organic chemistry video tutorial provides a basic introduction into the williamson ether synthesis reaction mechanism. It contains plenty of examples and practice problems.
Free Radical Reactions:
Reactions Summary:
Organic Chemistry 1 Final Exam Review:
IR Spectroscopy:
Mass Spectrometry:
______________________________
Proton NMR Spectroscopy:
Carbon-13 NMR Spectroscopy:
Ethers and Epoxides:
Diels Alder Reaction:
Organic Chemistry 2 Final Exam Review:
Free Radical Reactions:
Reactions Summary:
Organic Chemistry 1 Final Exam Review:
IR Spectroscopy:
Mass Spectrometry:
______________________________
Proton NMR Spectroscopy:
Carbon-13 NMR Spectroscopy:
Ethers and Epoxides:
Diels Alder Reaction:
Organic Chemistry 2 Final Exam Review:
Williamson Ether Synthesis Reaction Mechanism
Williamson Ether Synthesis
Williamson Ether Synthesis
Williamson ether synthesis
Williamson Ether Synthesis
Williamson Ether Synthesis
Williamson Ether Synthesis: Alkoxide + Primary Haloalkane
13.2 Synthesis of Ethers | Organic Chemistry
Williamson Ether Synthesis
The Williamson Ether Synthesis | Chemical reactions animation
A Brief Explanation of the Williamson Ether Synthesis
Williamson Ether Synthesis
Ether and Epoxide Reactions
Williamson Ether Synthesis Mechanism 003
Trick to write the product of Williamson's ether synthesis.
How to do Williamson Ether Synthesis - Mechanism/Product - NaOH , NaH - Organic Chemistry
Williamson Ether Synthesis
Williamson Ether Synthesis Reaction With Mechanism
Williamson Ether Synthesis MECHANISMS
Alcohols , Phenols n Ethers 13 :Preparation Of Ethers -Dehydration of Alcohol & Williamson Synth...
Williamson ether synthesis of ETHER || SN2|| Preparation of ether
Ether (R-O-R) Preparation by Dehydration of Alcohol & by Williamson Synthesis || NEET JEE | L-32
Williamson Ether Synthesis | | Step Wise Basic Mechanism of the reaction |Preparation of Ethers
Williamson ether synthesis in organic chemistry | IIT JEE & NEET | Vineet Khatri Sir | ATP STAR ...
Комментарии
 0:18:03
0:18:03
 0:07:49
0:07:49
 0:06:18
0:06:18
 0:02:26
0:02:26
 0:11:49
0:11:49
 0:01:32
0:01:32
 0:06:21
0:06:21
 0:05:17
0:05:17
 0:02:45
0:02:45
 0:02:42
0:02:42
 0:08:38
0:08:38
 0:09:44
0:09:44
 0:11:10
0:11:10
 0:07:39
0:07:39
 0:10:49
0:10:49
 0:02:37
0:02:37
 0:05:46
0:05:46
 0:06:38
0:06:38
 0:12:10
0:12:10
 0:46:30
0:46:30
 0:03:45
0:03:45
 0:25:11
0:25:11
 0:17:11
0:17:11
 0:07:36
0:07:36