filmov
tv
Kinetics: Activation Energy Determination from Experiment
Показать описание
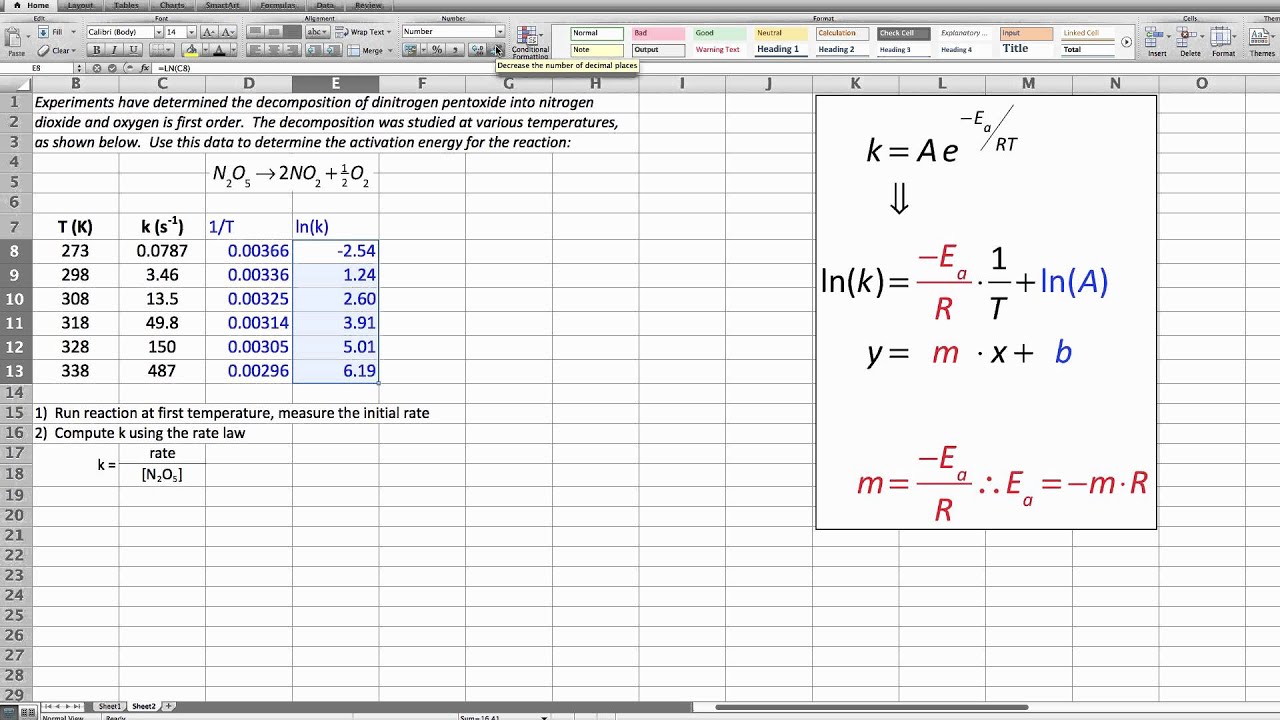
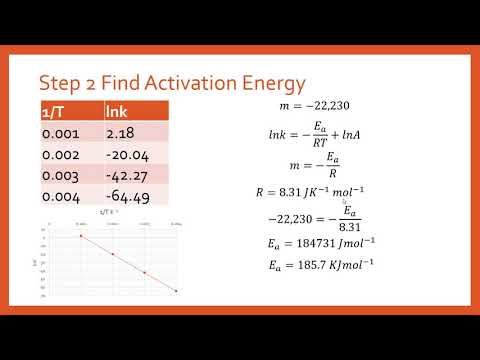
The activation energy of a reaction is determined by graphical means using experimental data.
Kinetics: Activation Energy Determination from Experiment
Activation Energy
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics
ACTIVATION ENERGY IN CHEMICAL KINETICS Pre-Lab - NYB Chemistry of Solutions
Chemistry - Chemical Kinetics (25 of 30) Determining the Activation Energy
Activation energy of an iodine clock reaction
16.3.2 Determine activation energy (Ea) values from the Arrhenius equation by a graphical method.
CHEMICAL KINETICS -2
Determination of energy of activation
KAC25.17 - Rates II: Experimental Determination of Arrhenius Parameters
The Arrhenius Equation
Determination of Activation Energy
To Calculate the Activation Energy from Thermogravimetric analysis data using Origin Software
How to Determine Activation Energy Using Nonlinear Regression
How to Use an Arrhenius Plot To Calculate Activation Energy and Intercept
⚗️ Using an Arrhenius Plot to Determine Kinetic Parameters
How to make an Arrhenius plot
How to Calculate Activation Energy (Ea) with Arrhenius Equation
14.4 Collision Theory and the Arrhenius Equation | General Chemistry
Chemical Kinetics: Determination of activation energy
Determination Of Activation Energy//Energy of activation
Using Arrhenius plot to determine Activation energy and Arrhenius constant | Chemical Kinetics
BSc.2 PBZ Prac.Chem.Chemical kinetics No.1
Chemical kinetics: To determine the energy of activation of a reaction between K2S2O8 and KI
Комментарии
 0:08:28
0:08:28
 0:04:52
0:04:52
 0:31:50
0:31:50
 0:03:02
0:03:02
 0:05:59
0:05:59
 0:04:12
0:04:12
 0:02:13
0:02:13
 0:38:07
0:38:07
 0:00:20
0:00:20
 0:04:52
0:04:52
 0:05:41
0:05:41
 0:08:18
0:08:18
 0:21:23
0:21:23
 0:04:24
0:04:24
 0:05:32
0:05:32
 0:07:22
0:07:22
 0:01:07
0:01:07
 0:15:23
0:15:23
 0:23:20
0:23:20
 0:01:01
0:01:01
 0:04:33
0:04:33
 0:08:29
0:08:29
 0:14:30
0:14:30
 0:01:52
0:01:52