filmov
tv
Hydrogen Bonding in Water

Показать описание
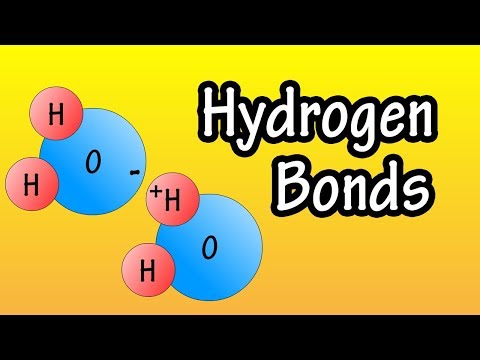
To understand hydrogen bonding in water we need to know that water is a polar molecule. This means that it has a positive and a negative "side".
Because the O atom in H2O is more electronegative the shared electrons will spend more time closer to the O. Since electrons are negative this means the O atom will have a negative charge. Therefore the H atoms will be positive.
Hydrogen bonding occurs when the negative side of one water molecule is attracted to the positive side of another molecule. This attraction forms a hydrogen bond.
Hydrogen bonds are weak compared to covalent and ionic bonds. Despite being weak they result in may of the unique properties associated with water. For example surface tension, cohesion, and a higher boiling point.
Because the O atom in H2O is more electronegative the shared electrons will spend more time closer to the O. Since electrons are negative this means the O atom will have a negative charge. Therefore the H atoms will be positive.
Hydrogen bonding occurs when the negative side of one water molecule is attracted to the positive side of another molecule. This attraction forms a hydrogen bond.
Hydrogen bonds are weak compared to covalent and ionic bonds. Despite being weak they result in may of the unique properties associated with water. For example surface tension, cohesion, and a higher boiling point.
Hydrogen Bonds - What Are Hydrogen Bonds - How Do Hydrogen Bonds Form
Hydrogen Bonds In Water Explained - Intermolecular Forces
Hydrogen Bonding in Water
Hydrogen bonding in water | Water, acids, and bases | Biology | Khan Academy
How Water Forms Hydrogen Bonds
How polarity makes water behave strangely - Christina Kleinberg
Hydrogen Bonding of Water Molecules | Chemistry
Hydrogen bonds in Water Molecules
Properties of Water | Hydrogen Bonding in Water | Biology | Biochemistry
Water and Hydrogen Bonding
Hydrogen bonds / hydrogen bonding in water / the basics
Water and Hydrogen Bonds (The Importance for Living Organisms)
Why does ice float in water? - George Zaidan and Charles Morton
How many hydrogen bonds a water molecule can form | Hydrogen Bonding in Water molecules
Polar Bonds and Hydrogen Bonds
How many hydrogen bonds can a water molecule form?
Properties of Water
Hydrogen Bonding | Chemistry
(2.2) - HYDROGEN BONDING In Water - (IB Biology) - TeachMe
007-Hydrogen Bonding & Water
The Structure of Water and Hydrogen Bonding | AP Biology 1.1
Hydrogen bonding in water
Extensive Hydrogen Bonding | Chapter # 15 | Chemistry Class 10th | Lec #
1.1 Structure of Water and Hydrogen Bonding - AP Biology
Комментарии
 0:02:48
0:02:48
 0:10:54
0:10:54
 0:04:36
0:04:36
 0:06:46
0:06:46
 0:11:40
0:11:40
 0:03:52
0:03:52
 0:03:57
0:03:57
 0:01:06
0:01:06
 0:12:37
0:12:37
 0:05:06
0:05:06
 0:02:17
0:02:17
 0:06:01
0:06:01
 0:03:56
0:03:56
 0:04:57
0:04:57
 0:02:37
0:02:37
 0:01:30
0:01:30
 0:06:51
0:06:51
 0:06:31
0:06:31
 0:12:36
0:12:36
 0:09:01
0:09:01
 0:14:13
0:14:13
 0:01:01
0:01:01
 0:11:26
0:11:26
 0:10:14
0:10:14