filmov
tv
Frost Circles

Показать описание
Frost Circles
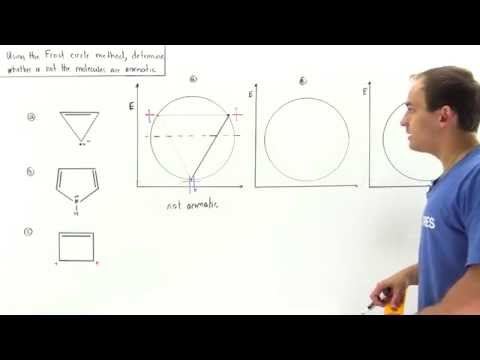
Frost Circle Method Example
Frost Circles, Hückel's Rule and Aromaticity
Celtic Frost – Circle Of The Tyrants (Official HD Video)
Frost Circle Method
Frost Circles
Frost Circles and 4n + 2 rule
17.4 Aromaticity and Molecular Orbital Theory
Frost circles
14.2 Frost’s Circle
Frost circles - approximating molecular orbitals
Chem 2060 (F20) - Project #5, Frost Circles Explanation
31.06 The Frost Circle
18.9 - Using Frost Circles to Decide Whether a Molecule is Non-Aromatic, Aromatic, or Anti-Aromatic
Chem 2060 (F20) - Project #5, Cyclobutadiene Frost Circle Tutorial
Chapter 14 - Frost Circles
AROMATICITY | FROST CIRCLES DIAGRAM | CSIR-NET | GATE | IIT-JAM | SET | MSC-ENTRANCES
Frost Musulin Diagram | Stability of aromatic and anti-aromatic compounds
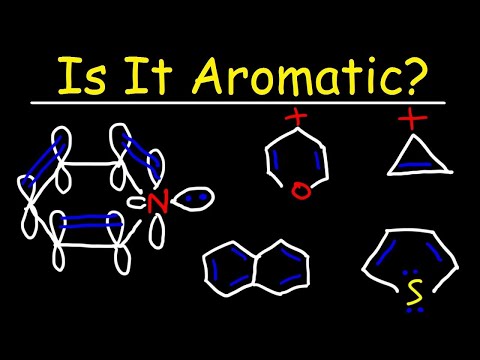
Aromatic, Antiaromatic, or Nonaromatic - Huckel's Rule - 4n+2 - Heterocycles
Circle Theorems - GCSE
benzene frost circle trig
Aromaticity 2: Frost Circles
MY REAL EYEBALL 😳 #shorts
Organic Chemistry 2 - Ch17.9 - Frost Circles
Комментарии
 0:09:42
0:09:42
 0:09:46
0:09:46
 0:08:04
0:08:04
 0:04:37
0:04:37
 0:11:06
0:11:06
 0:05:12
0:05:12
 0:03:10
0:03:10
 0:08:24
0:08:24
 0:06:45
0:06:45
 0:16:18
0:16:18
 0:01:56
0:01:56
 0:01:05
0:01:05
 0:04:17
0:04:17
 0:05:30
0:05:30
 0:02:40
0:02:40
 0:08:54
0:08:54
 0:12:08
0:12:08
 0:11:12
0:11:12
 0:10:43
0:10:43
 0:18:18
0:18:18
 0:02:18
0:02:18
 0:13:50
0:13:50
 0:00:21
0:00:21
 0:06:18
0:06:18