filmov
tv
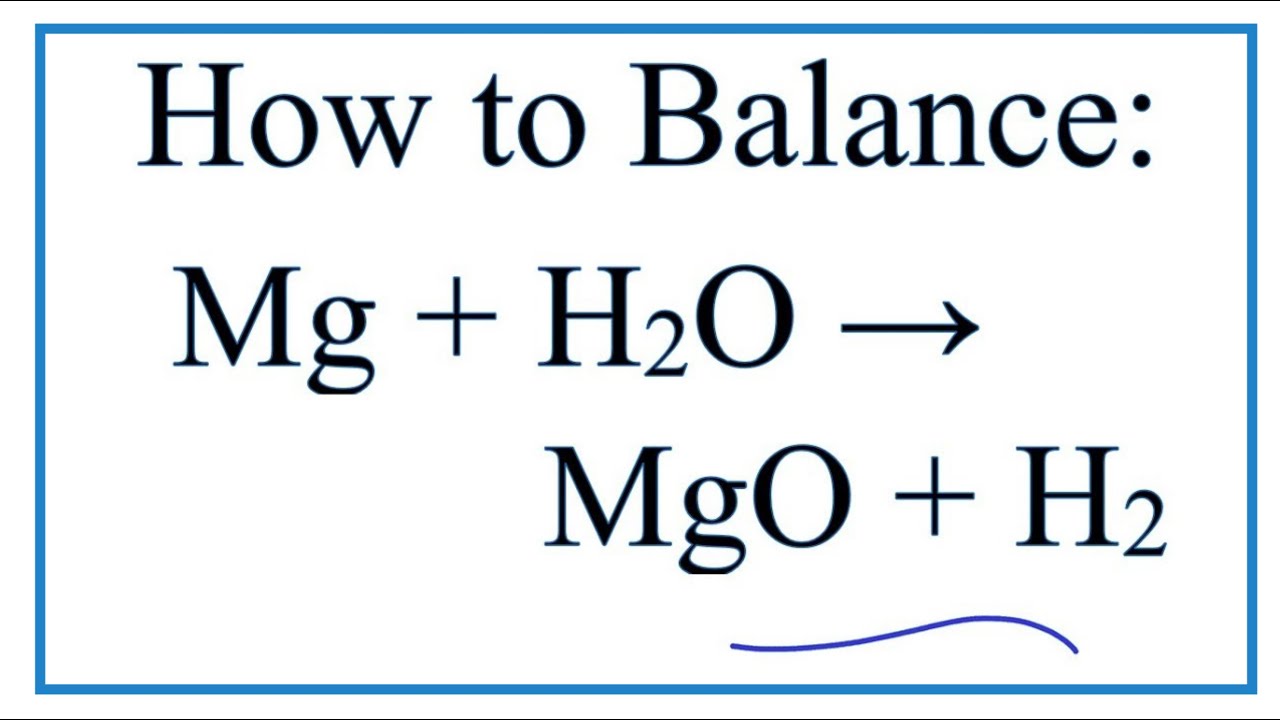
How to Balance Mg + H2O = MgO + H2 | Magnesium + Water (steam)
Показать описание
In this video we'll balance the equation Mg + H2O = MgO + H2 and provide the correct coefficients for each compound.
To balance Mg + H2O = MgO + H2 you'll need to be sure to count all of atoms on each side of the chemical equation.
Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Magnesium + Water (steam).
Important tips for balancing chemical equations:
Only change the numbers in front of compounds (the coefficients).
Never change the numbers after atoms (the subscripts).
The number of each atom on both sides of the equation must be the same for the equation to be balanced.
For a complete tutorial on balancing all types of chemical equations, watch my video:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
To balance Mg + H2O = MgO + H2 you'll need to be sure to count all of atoms on each side of the chemical equation.
Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Magnesium + Water (steam).
Important tips for balancing chemical equations:
Only change the numbers in front of compounds (the coefficients).
Never change the numbers after atoms (the subscripts).
The number of each atom on both sides of the equation must be the same for the equation to be balanced.
For a complete tutorial on balancing all types of chemical equations, watch my video:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
How to Balance Mg + O2 = MgO (Magnesium plus Oxygen Gas)
How to Balance Mg + N2 = Mg3N2
How to Balance Mg + H2O = MgO + H2 | Magnesium + Water (steam)
How to Balance Mg + HCl → MgCl2 + H2 (Magnesium + Hydrochloric Acid)
How to Balance Mg(OH)2 + HCl = MgCl2 + H2O (Magnesium hydroxide + Hydrochloric acid)
How to Balance Mg + H2O = Mg(OH)2 + H2 (Magnesium Metal plus Water)
How to Balance Mg + Cl2 = MgCl2 (Magnesium + Chlorine gas)
How to Balance Mg(OH)2 + HNO3 = Mg(NO3)2 + H2O (Magnesium hydroxide + Nitric acid)
321FY4 MY23 MG ZS Excite
How to Balance Mg + H2SO4 = MgSO4 + H2 (Magnesium + Dilute Sulfuric acid
How to Balance MgO + H2O = Mg(OH)2 (Magnesium Oxide plus Water)
How to Balance Mg + CO2 = MgO + C (Magnesium plus Carbon Dioxide)
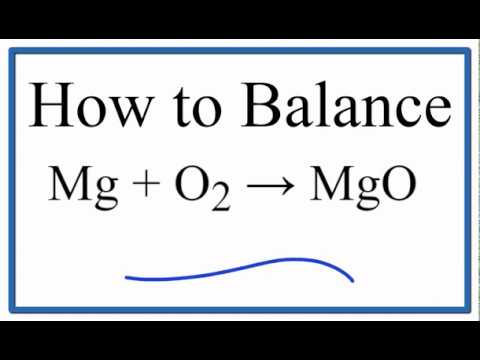
How to balance: Mg + O2 = MgO
How to balance Mg + O2 = MgO
How to Balance Mg + Fe2O3 = Fe + MgO (Magnesium + Iron (III) Oxide)
Balancing :Mg + O2 → MgO
How to Balance Mg + HCl → MgCl2 + H2 (Single Displacement) #shorts
Electrolyte Imbalances (Na, Ca, K, Mg) - Medical-Surgical - Cardiovascular | @LevelUpRN
How to Balance Mg + P4 = Mg3P2 (Magnesium + Tetraphosphorous)
How to Balance Mg + CuSO4 = Cu + MgSO4
How to Balance Mg(NO3)2 = MgO + NO2 + O2 (Magnesium nitrate)
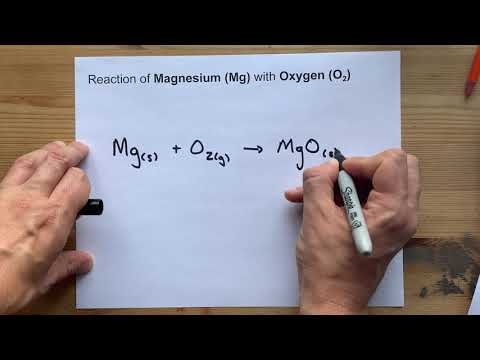
Reaction of Magnesium with Oxygen ... Mg + O2 =
How to Balance Mg + AgNO3 = Mg(NO3)2 + Ag (Magnesium + Silver nitrate)
How to Balance HNO3 + Mg = Mg(NO3)2 + H2 (note: Dilute HNO3)
Комментарии
 0:01:02
0:01:02
 0:01:00
0:01:00
 0:00:50
0:00:50
 0:01:16
0:01:16
 0:02:05
0:02:05
 0:01:22
0:01:22
 0:00:54
0:00:54
 0:02:28
0:02:28
 0:02:09
0:02:09
 0:01:15
0:01:15
 0:01:04
0:01:04
 0:01:35
0:01:35
 0:01:20
0:01:20
 0:02:23
0:02:23
 0:01:17
0:01:17
 0:01:34
0:01:34
 0:00:55
0:00:55
 0:16:27
0:16:27
 0:01:11
0:01:11
 0:01:11
0:01:11
 0:02:28
0:02:28
 0:00:41
0:00:41
 0:01:30
0:01:30
 0:01:14
0:01:14