filmov
tv
Example of a Precipitation Reaction

Показать описание
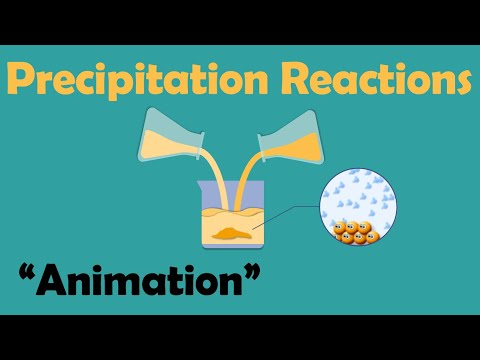
Precipitation reactions occur when two aqueous solutions are mixed together and a solid forms. The solid is called a precipitate since it falls to the bottom of the test-tube or beaker.
The reactants are dissolved in water so we have an aqueous solution. In the water they will split apart (dissociated) into their ions. If the ions can combine to form an insoluble substance this will be the precipitate in the reaction.
The keys for determine is an reaction is an example of precipitation:
1. Identify the pattern.
2. Write the ionic charges above.
3. Write products.
4. Balance charges for products.
5. Check solubility and write the states.
Chapters:
00:00 Intro
00:24 Example of a Precipitation Reaction
00:44 Write Ionic Charges
01:20 Predicting the Products
01:50 Check Solubility of Products
2:44 Review Precipitation Reaction
Solubility Resources (tools to tell if a precipitation reaction occurs):
These are some of the basic rules for solubility
- Group I elements (Li+, Na+, K+, Cs+, Rb+) are
- NH4+ (Ammonium ion) are soluble.
- the nitrate ion (NO3-) are generally soluble.
- of Cl-, Br-, and I- are soluble. Exceptions Ag+, Pb2+, and (Hg2)2+
- most sulfates are soluble. Exceptions: Ba2+, Ca2+, Pb2+, Ag+, Sr2+
- most hydroxide salts are only slightly soluble. Exceptions: NH4+, Li+, Na+, K+
- most carbonates (CO32-) are insoluble. Exceptions: Group 1 and NH4+
Note: Rules at the top supersede any lower rules.
---
ns.
The reactants are dissolved in water so we have an aqueous solution. In the water they will split apart (dissociated) into their ions. If the ions can combine to form an insoluble substance this will be the precipitate in the reaction.
The keys for determine is an reaction is an example of precipitation:
1. Identify the pattern.
2. Write the ionic charges above.
3. Write products.
4. Balance charges for products.
5. Check solubility and write the states.
Chapters:
00:00 Intro
00:24 Example of a Precipitation Reaction
00:44 Write Ionic Charges
01:20 Predicting the Products
01:50 Check Solubility of Products
2:44 Review Precipitation Reaction
Solubility Resources (tools to tell if a precipitation reaction occurs):
These are some of the basic rules for solubility
- Group I elements (Li+, Na+, K+, Cs+, Rb+) are
- NH4+ (Ammonium ion) are soluble.
- the nitrate ion (NO3-) are generally soluble.
- of Cl-, Br-, and I- are soluble. Exceptions Ag+, Pb2+, and (Hg2)2+
- most sulfates are soluble. Exceptions: Ba2+, Ca2+, Pb2+, Ag+, Sr2+
- most hydroxide salts are only slightly soluble. Exceptions: NH4+, Li+, Na+, K+
- most carbonates (CO32-) are insoluble. Exceptions: Group 1 and NH4+
Note: Rules at the top supersede any lower rules.
---
ns.
Комментарии
 0:00:35
0:00:35
 0:00:57
0:00:57
 0:00:35
0:00:35
 0:03:29
0:03:29
 0:10:14
0:10:14
 0:00:30
0:00:30
 0:00:16
0:00:16
 0:04:55
0:04:55
 0:07:51
0:07:51
 0:11:31
0:11:31
 0:04:08
0:04:08
 0:01:23
0:01:23
 0:06:54
0:06:54
 0:00:08
0:00:08
 0:00:42
0:00:42
 0:24:04
0:24:04
 0:00:43
0:00:43
 0:08:20
0:08:20
 0:05:05
0:05:05
 0:02:18
0:02:18
 0:12:51
0:12:51
 0:10:52
0:10:52
 0:07:43
0:07:43
 0:03:36
0:03:36