filmov
tv
Carboxylic Acids

Показать описание
Another family of chemicals with a carbonyl group in. Take a peek at this video to find out how to name carboxylic acids, physical properties and chemical reactions with metals, bases and carbonates. #usefulacids
GCSE Chemistry - Carboxylic Acids
Carboxylic Acids: Crash Course Organic Chemistry #30
Carboxylic Acids, Typical Acids and Esters | Organic Chemistry | Chemistry | FuseSchool
Carboxylic acid introduction | Carboxylic acids and derivatives | Organic chemistry | Khan Academy
GCSE Chemistry Revision 'Carboxylic Acids' (Triple)
Naming Carboxylic Acids - IUPAC Nomenclature
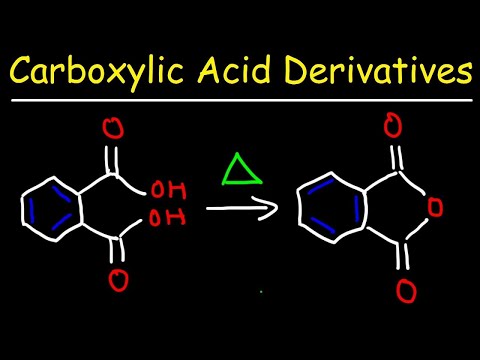
Carboxylic Acid Derivative Reactions
Carboxylic Acids and Their Derivatives
Carboxylic Acids & Their Derivatives | Decarboxylation & Reduction Reactions | AskPrep
Tests for Carboxylic Acids - MeitY OLabs
20.1 Naming Carboxylic Acids and Acid Derivatives | Organic Chemistry
Ch#20 | Lec#1 | Carboxylic Acids and Functional Derivatives, methods of preparation, Physical prop
Carboxylic Acid Derivatives & Hydrolysis Reactions: Crash Course Organic Chemistry #31
Testing For Carboxylic Acids | Sodium Carbonate
A-Level Chemistry - What are Carboxylic Acids?
Carboxylic Acids | Organic Chemistry
AQA 3.9 Carboxylic Acids and Derivatives REVISION
Plus Two Chemistry | Aldehydes Ketones and Carboxylic Acids | Full Chapter | Exam Winner
Carboxylic Acids
Carboxylic Acid Derivatives Overview and Reaction Map
Carboxylic Acids Grade 12 Chemistry: All about carboxylic acids & naming carboxylic acids
Naming Carboxylic Acids - Organic Chemistry IUPAC Naming by Leah4sci
Plus Two Chemistry | Aldehydes , Ketones And Carboxylic Acids | Xylem Plus Two
OCR A 6.1.3 Carboxylic Acids and Esters REVISION
Комментарии
 0:03:05
0:03:05
 0:11:36
0:11:36
 0:04:30
0:04:30
 0:08:51
0:08:51
 0:03:41
0:03:41
 0:14:31
0:14:31
 0:10:49
0:10:49
 0:13:40
0:13:40
 0:07:18
0:07:18
 0:03:20
0:03:20
 0:10:28
0:10:28
 0:28:26
0:28:26
 0:12:27
0:12:27
 0:00:16
0:00:16
 0:07:35
0:07:35
 0:09:59
0:09:59
 1:04:14
1:04:14
 3:06:02
3:06:02
 0:08:04
0:08:04
 0:10:10
0:10:10
 0:08:39
0:08:39
 0:10:18
0:10:18
 3:32:21
3:32:21
 0:26:05
0:26:05