filmov
tv
All of AQA Atomic Structure and Radiation Explained - GCSE Physics 9-1 REVISION

Показать описание
This video is a summary of all of AQA Atomic Structure and Radiation, explained for GCSE Physics 9-1. You can use this as an AQA Atomic Structure and Radiation revision tool.
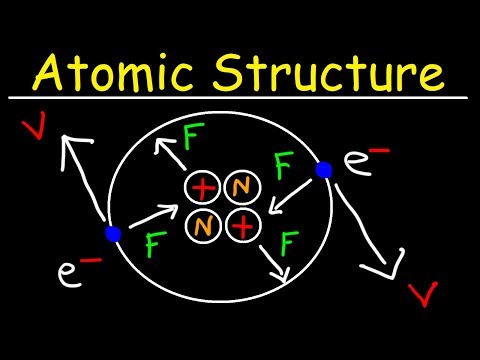
We start with the structure of the atom: a dense, positive nucleus containing protons and neutrons surrounded by shells of negative electrons. This was discovered using the Alpha Scattering Experiment. The atomic number is the number of protons as well as the number of electrons and the mass number is the total number of protons and neutrons.
An isotope of an element has the same number of protons but a different number of neutrons. Isotopes can be unstable and undergo radioactive decay. There are different types of radioactive decay including: alpha radiation, beta radiation, gamma radiation, neutron emission and positron emission (beta+). The atomic and mass numbers must be conserved in radioactive decay.
The activity of a radioactive isotope is the amount of radioactive decays per second, measured in becquerels (Bq). The activity of an isotope exponentially decays with time. This means that it decreases by the same proportion after each time period. The time it takes for the activity of an isotope to halve is the half-life.
Radiation can be dangerous. However it can also be useful and is used in medicine. It can be used to locate as well as treating problem areas in the human body. Nuclear decay (fission) releases huge amounts of energy and can be used to generate electricity.
Thanks for watching,
Lewis
Relevant for GCSE Physics 9-1 in the following exam boards:
AQA (including Trilogy)
_____________________________________
MY PHYSICS WEBSITES
Find even more videos organised by exam board and topic at:
GCSE Physics Online
A Level Physics Online
MY YOUTUBE CHANNEL
Your support in watching this video has been invaluable! To contribute towards the free videos on YouTube, make a small donation at:
FOLLOW ME
#radiation #gcsephysics #physicsonline
We start with the structure of the atom: a dense, positive nucleus containing protons and neutrons surrounded by shells of negative electrons. This was discovered using the Alpha Scattering Experiment. The atomic number is the number of protons as well as the number of electrons and the mass number is the total number of protons and neutrons.
An isotope of an element has the same number of protons but a different number of neutrons. Isotopes can be unstable and undergo radioactive decay. There are different types of radioactive decay including: alpha radiation, beta radiation, gamma radiation, neutron emission and positron emission (beta+). The atomic and mass numbers must be conserved in radioactive decay.
The activity of a radioactive isotope is the amount of radioactive decays per second, measured in becquerels (Bq). The activity of an isotope exponentially decays with time. This means that it decreases by the same proportion after each time period. The time it takes for the activity of an isotope to halve is the half-life.
Radiation can be dangerous. However it can also be useful and is used in medicine. It can be used to locate as well as treating problem areas in the human body. Nuclear decay (fission) releases huge amounts of energy and can be used to generate electricity.
Thanks for watching,
Lewis
Relevant for GCSE Physics 9-1 in the following exam boards:
AQA (including Trilogy)
_____________________________________
MY PHYSICS WEBSITES
Find even more videos organised by exam board and topic at:
GCSE Physics Online
A Level Physics Online
MY YOUTUBE CHANNEL
Your support in watching this video has been invaluable! To contribute towards the free videos on YouTube, make a small donation at:
FOLLOW ME
#radiation #gcsephysics #physicsonline
Комментарии
 0:13:09
0:13:09
 0:21:14
0:21:14
 0:05:22
0:05:22
 0:14:23
0:14:23
 0:01:00
0:01:00
 0:07:54
0:07:54
 0:04:32
0:04:32
 0:00:49
0:00:49
 0:15:48
0:15:48
 0:33:00
0:33:00
 0:36:52
0:36:52
 0:07:20
0:07:20
 0:30:37
0:30:37
 1:14:48
1:14:48
 0:27:47
0:27:47
 1:13:47
1:13:47
 0:19:56
0:19:56
 0:11:45
0:11:45
 0:24:36
0:24:36
 0:00:20
0:00:20
 0:09:10
0:09:10
 0:01:00
0:01:00
 0:00:58
0:00:58
 0:40:09
0:40:09