filmov
tv
Why does water evaporate at room temperature?

Показать описание
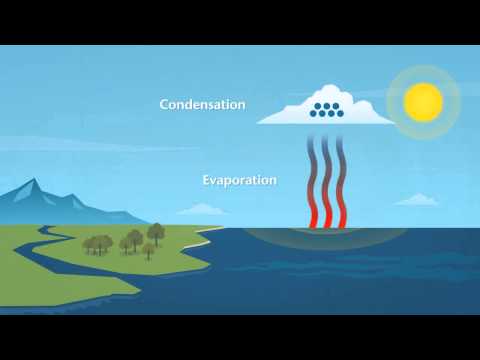
Water evaporates at room temperature because a portion of the water will have enough energy to become a gas at any given time. Over enough time, then all the water will evaporate.
0:00 - Intro
1:02 - Temperature
1:27 - States of matter
1:45 - Some water has enough energy
2:22 - Water at room temperature
3:18 - Moving Air
3:36 - Escaping water decreases temperature?
4:20 - Main point
4:34 - Outro
#BiscuitPhysics #Physics #Water
0:00 - Intro
1:02 - Temperature
1:27 - States of matter
1:45 - Some water has enough energy
2:22 - Water at room temperature
3:18 - Moving Air
3:36 - Escaping water decreases temperature?
4:20 - Main point
4:34 - Outro
#BiscuitPhysics #Physics #Water
Why does water evaporate at room temperature?
Why Does Water Evaporate at Room Temperature?
How Does Water Evaporate on its Own?
How does water evaporate?
How Does Water Evaporate? | PLUM LANDING on PBS KIDS
How long does water evaporate at room temperature?
Why does ocean water evaporate even though it does not reach a boiling point ?
How does water evaporate if it never reaches boiling point?
The Water Cycle | The Dr. Binocs Show | Learn Videos For Kids
How does water evaporate without boiling?
Does water evaporate faster as temperature rises?
why do water Evaporate?
How long does it take water to evaporate when boiling?
Why does water evaporate at room temp?
Why does water evaporate at 100 degrees?
How does rain form and what is the water cycle?
Science - How long does it take for water to evaporate?
Boiling Water Without Heat | Earth Science
Can water evaporate at any temperature?
Does water evaporate at 25 C?
Why does water evaporate faster in winter?
Why does water evaporate before 100 degrees?
The Water Cycle for Kids | Learn all about the water cycle
Why can water evaporate at any temperature?
Комментарии
 0:05:03
0:05:03
 0:03:24
0:03:24
 0:03:14
0:03:14
 0:06:49
0:06:49
 0:02:40
0:02:40
 0:00:55
0:00:55
 0:04:25
0:04:25
 0:01:00
0:01:00
 0:03:09
0:03:09
 0:01:12
0:01:12
 0:00:53
0:00:53
 0:00:13
0:00:13
 0:00:48
0:00:48
 0:00:34
0:00:34
 0:00:45
0:00:45
 0:01:48
0:01:48
 0:01:19
0:01:19
 0:02:58
0:02:58
 0:00:43
0:00:43
 0:00:37
0:00:37
 0:00:35
0:00:35
 0:00:47
0:00:47
 0:07:13
0:07:13
 0:00:39
0:00:39