filmov
tv
Astronomy - Ch. 5: Light & E&M Radiation (21 of 30) Emission Spectrum of Nebula
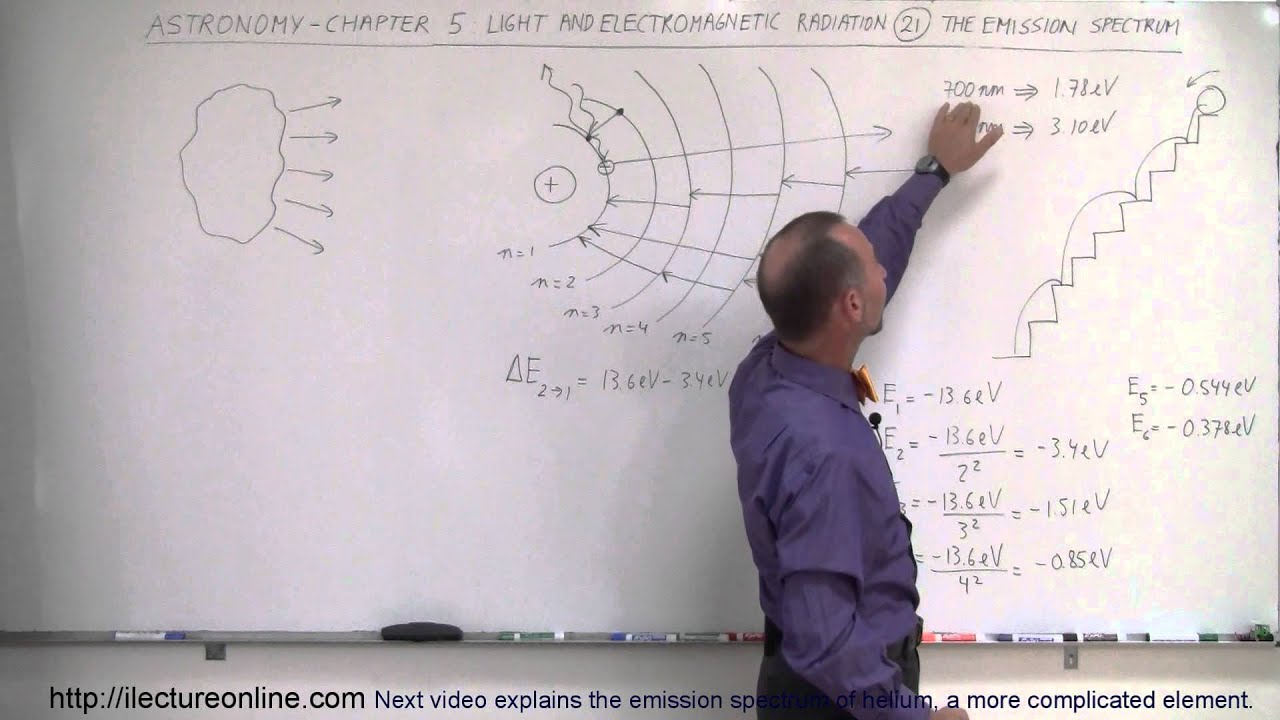
Показать описание
In this video I will explain why nebula have emission spectra when they are not very hot.
Astronomy - Ch. 5: Light & E&M Radiation (1 of 30) Types of Radiation
Astronomy - Ch. 5: Light & E&M Radiation (12 of 30) What is Light made Of?
Astronomy - Ch. 5: Light & E&M Radiation (10 of 30) Differences: Transparent vs Opaque Atmos...
Astronomy - Ch. 5: Light & E&M Radiation (20 of 30) Why Light Sources have Different Spectra...
Astronomy - Ch. 5: Light & E&M Radiation (5 of 30) How Are E&M Waves Produced?
Astronomy Chapter 5
Astronomy - Ch. 5: Light & E&M Radiation (7 of 30) What Are Frequencies of Light?
Astronomy - Ch. 5: Light & E&M Radiation (3 of 30) Important Basics Of Waves
5 Mind-Blowing Space Facts You Won’t Believe! #astronomy #mindblowing #spacefacts #spacemysteries
Astronomy - Ch. 5: Light & E&M Radiation (30 of 30) What Can Observing E&M Radiation Tel...
Astronomy - Ch. 5: Light & E&M Radiation (6 of 30) Difference of E&M Frequence
Astronomy - Ch. 5: Light & E&M Radiation (21 of 30) Emission Spectrum of Nebula
Astronomy - Ch. 5: Light & E&M Radiation (4 of 30) What Produces E&M Waves?
Astronomy - Ch. 5: Light & E&M Radiation (23 of 30) Emission Spectrum and Amount of Elements
Astronomy - Ch. 5: Light & E&M Radiation (26 of 30) Doppler Shift and Celestial Radial Movem...
Astronomy - Ch. 5: Light & E&M Radiation (27 of 30) Red Shift and Blue Shift Explained
Astronomy - Ch. 5: Light & E&M Radiation (14 of 30) Why is UV Radiation Dangerous?
Astronomy - Ch. 5: Light & E&M Radiation (24 of 30) Emission Spectrum of Celestial Object
Astronomy - Ch. 5: Light & E&M Radiation (8 of 30) E&M Radiation...Safe or Dangerous?
Astronomy - Ch. 5: Light & E&M Radiation (19 of 30) Why Do Hot Objects Glow?
Astronomy Chapter 5 Lecture Video
Astronomy - Ch. 5: Light & E&M Radiation (2 of 30) Differences in Radiation
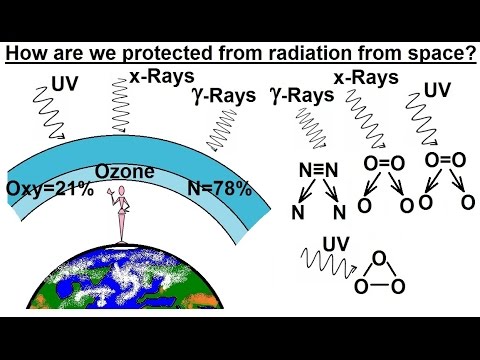
Astronomy - Ch. 5: Light & E&M Radiation (9 of 30) How Does the Atmosphere Protect Us?
Astronomy - Ch. 5: Light & E&M Radiation (28 of 30) Finding the Velocities of Celestial Obje...
Комментарии
 0:03:39
0:03:39
 0:07:00
0:07:00
 0:03:20
0:03:20
 0:05:00
0:05:00
 0:09:25
0:09:25
 0:13:30
0:13:30
 0:03:00
0:03:00
 0:04:12
0:04:12
 0:01:23
0:01:23
 0:04:12
0:04:12
 0:03:49
0:03:49
 0:13:11
0:13:11
 0:02:34
0:02:34
 0:02:06
0:02:06
 0:03:11
0:03:11
 0:04:44
0:04:44
 0:03:37
0:03:37
 0:07:15
0:07:15
 0:05:35
0:05:35
 0:02:44
0:02:44
 0:12:02
0:12:02
 0:05:08
0:05:08
 0:04:22
0:04:22
 0:03:14
0:03:14