filmov
tv
What stereoisomerism does this alkene have? #chemistry #shorts

Показать описание
#chemistry #alevels #organicchemistry #alevelchemistry
E/Z Absolute Configuration of Alkenes
What stereoisomerism does this alkene have? #chemistry #shorts
Cis and Trans Isomers
Alkene stereoisomers
Stereoisomers
Using Cis/Trans versus E/Z to Describe Double Bonds
Naming Alkenes Using E Z System - IUPAC Nomenclature
E Z Geometric Isomers for Alkenes
Isomerism -7 | Lecture -27
Structural Isomers and Stereoisomers
Cis–trans isomerism | Alkenes and Alkynes | Organic chemistry | Khan Academy
20.6.2 Geometric Isomerism in Alkenes IB Chemistry HL
alkene geometry in stereochemistry
in seconds you can find isomers #isomers #alkane #alkene for alkynes watch next video#trickforisomer
Alkene: Isomerism
•Stereoisomers in organic chemistry: Geometrical isomers in alkene
Isomerism (Definition, Types and Examples)
4.1.3 alkene stereoisomerism | A Level Chemistry
Alkene Stereoisomerism - #shorts #chemistry #shortvideo
How to Find Possible Isomers of a Compound? Trick to Find Isomers
Isomers | Organic Chemistry | A level
Isomers | Properties of carbon | Biology | Khan Academy
54 CHM2210 Alkene Stereoisomers
General Chemistry: Cis and Trans Isomers of Alkene
Комментарии
 0:06:01
0:06:01
 0:00:20
0:00:20
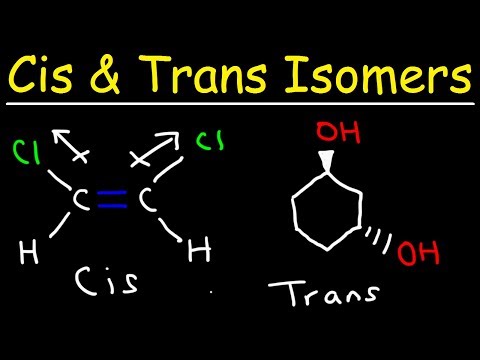 0:06:35
0:06:35
 0:12:10
0:12:10
 0:05:48
0:05:48
 0:15:21
0:15:21
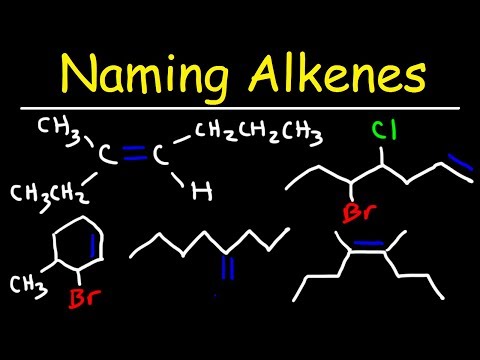 0:12:18
0:12:18
 0:12:07
0:12:07
 0:54:25
0:54:25
 0:03:30
0:03:30
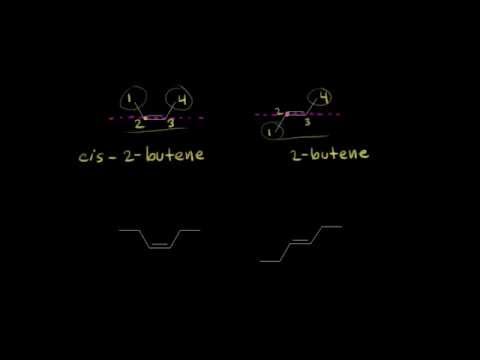 0:05:24
0:05:24
 0:00:51
0:00:51
 0:04:42
0:04:42
 0:00:09
0:00:09
 0:11:33
0:11:33
 0:14:13
0:14:13
 0:15:34
0:15:34
 0:18:02
0:18:02
 0:00:50
0:00:50
 0:02:41
0:02:41
 0:47:13
0:47:13
 0:06:48
0:06:48
 0:22:30
0:22:30
 0:11:16
0:11:16