filmov
tv
Periodic Table Trends Trick (Electronegativity, Atomic Radius, Ionization Energy, Electron Affinity)
Показать описание
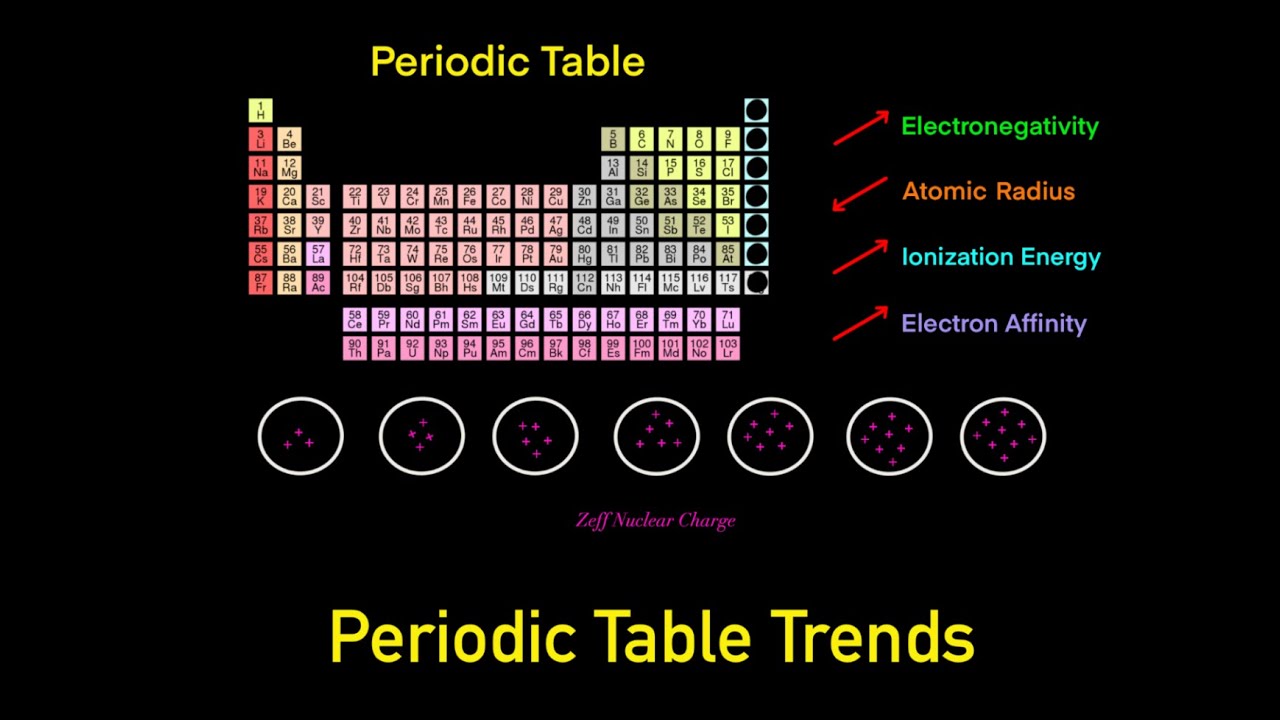
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Periodic Table Trends Trick (Electronegativity, Atomic Radius, Ionization Energy, Electron Affinity)
Tricks to Remember Periodic Trends
How to calculate Electronegativity? Easy Trick
Periodic Trends - Atomic Radius, Electronegativity, Ionization Energy - Chemistry Series
Electronegativity | Periodic Trends | Chemistry
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE
Best 💯TRICK 🔥💡to learn ELECTRONEGATIVITY values #jee #iitjee #jeemains #iit #tricks #trick #neet...
The Periodic Table Song.. ( credit goes to the rightful owner)
Periodic Table Trends : Atomic Radius, Electronegativity, Ionisation Energy ||Short Trick Just 5 Min
Periodic Table Trends: Electronegativity + Size 📈
Short trick to Learn Electronegativity Values 🧐🧐💪🏻⚡| Motion NEET | #neet #shorts #poonammam #tricks...
periodic table chemistry wali bhabhi ke yad Hain #chemistry
MCAT Mnemonic: Periodic Trends (Ep. 5)
Trick to remember Electronegativity#shorts #science #chemistry
Periodic Trends of the Periodic Table
Complete Periodic table in just 60 seconds 🔥🔥😮😯
Song Of chemistry 😂😂 #science #chemistry #songs
How the MCAT Tests - Periodic Table Trends & Physics Correlates
Best Trick🔥to remember Order of Ionisation Energy | #Iit #neet #shorts #hack
118 elements name in just 52 seconds #shorts_#118 elements
Valency Trick|NEET|JEE|#shorts #viral
How To Memorize Periodic Table? Super Easy Trick
Ionization Energy | Periodic Trends
Комментарии
 0:07:53
0:07:53
 0:15:53
0:15:53
 0:03:54
0:03:54
 0:06:30
0:06:30
 0:18:06
0:18:06
 0:10:00
0:10:00
 0:24:55
0:24:55
 0:03:03
0:03:03
 0:00:31
0:00:31
 0:09:52
0:09:52
 0:00:30
0:00:30
 0:01:00
0:01:00
 0:00:25
0:00:25
 0:01:59
0:01:59
 0:00:59
0:00:59
 0:12:34
0:12:34
 0:01:01
0:01:01
 0:00:14
0:00:14
 0:13:09
0:13:09
 0:01:01
0:01:01
 0:00:59
0:00:59
 0:00:32
0:00:32
 0:08:15
0:08:15
 0:10:59
0:10:59