filmov
tv
API Process Validation: How to reduce validation costs (wow)

Показать описание
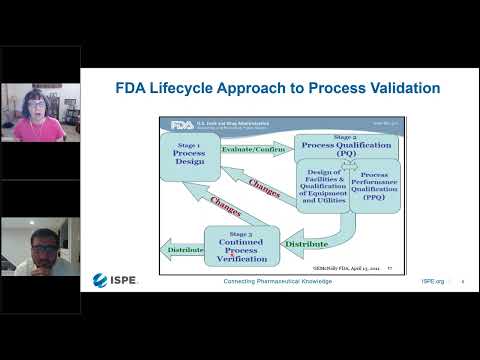
The FDA process validation guidance speaks to phase 1 being process design. When your drug substance process is designed is very robust, what does process validation entail? Non-critical synthesis steps are not required to be validated according to ICH Q7.
For API’s, the Pharmaceutical Quality Risk Management has a huge impact on process validation. Process validation is only one-way Quality Risk Management in the pharmaceutical industry is changing the compliance landscape.
The approach involves ensuring variation impacting Critical Quality Attributes is controlled. The validation covers all Critical Process Parameters identified in the Quality by Design process.
Follow our LinkedIn Business Page to ensure you see all of our valuable content.
For API’s, the Pharmaceutical Quality Risk Management has a huge impact on process validation. Process validation is only one-way Quality Risk Management in the pharmaceutical industry is changing the compliance landscape.
The approach involves ensuring variation impacting Critical Quality Attributes is controlled. The validation covers all Critical Process Parameters identified in the Quality by Design process.
Follow our LinkedIn Business Page to ensure you see all of our valuable content.
API Process Validation: How to reduce validation costs (wow)
Process Validation | Types of Process Validation | Process Performance Qualification
PROCESS VALIDATION || API INDUSTRY || SM PHARMA SOLUTIONS
Process Validation for API by Bhaskarsri
PROCESS VALIDATION STAGE-1 'PROCESS DESIGN'
PROCESS VALIDATION I PART-1 I INTRO I IMPORTANCE I HINDI
PROCESS VALIDATION IN PHARMACEUTICALS
Process Validation and ICH Q7
Mastering the UiPath Document Understanding Framework
Process Validation | PV and its Life cycle approach
API I PART-1 I INTRO I CATEGORIES I MANUFACTURING PROCESS
How to Write a Validation Protocol | Different Parts of Validation Protocol
Practical Application Points for Process Validation Lifecycle Approach
Lifecycle Approach to Process Validation
Process Validation I Definition l Types l Stages l Pharmaceutical Quality Assurance
Cleaning Validation - analytical demonstration
Why We Use Three Batches For Validation | Myth Of 3 Validation Batches
Validation in pharmaceutical industry I Interview Questions and Answers | hindi
FDA Pharmaceutical Validation Guidance and ICH: What you must know
Webinar: Modern Process Validation
How to know about Validation process in pharma industry in Telugu || Pharma Guide
Process Validation | Definition | Stages | Types | Pharmaceutical Quality Assurance | BP606T | L~51
process validation interdiction telugu explain. @ Pharma collection.
Process Validations details in Pharmaceuticals
Комментарии
 0:01:20
0:01:20
 0:08:50
0:08:50
 0:31:17
0:31:17
 1:03:22
1:03:22
 0:09:00
0:09:00
 0:25:42
0:25:42
 0:31:54
0:31:54
 0:21:07
0:21:07
 1:25:29
1:25:29
 0:11:25
0:11:25
 0:14:15
0:14:15
 0:03:17
0:03:17
 1:18:55
1:18:55
 2:04:33
2:04:33
 0:18:03
0:18:03
 0:01:35
0:01:35
 0:07:15
0:07:15
 0:09:45
0:09:45
 0:08:49
0:08:49
 0:52:58
0:52:58
 0:09:30
0:09:30
 0:31:34
0:31:34
 0:01:56
0:01:56
 0:25:36
0:25:36