filmov
tv
Structure of Sodium Chloride (NaCl)
Показать описание
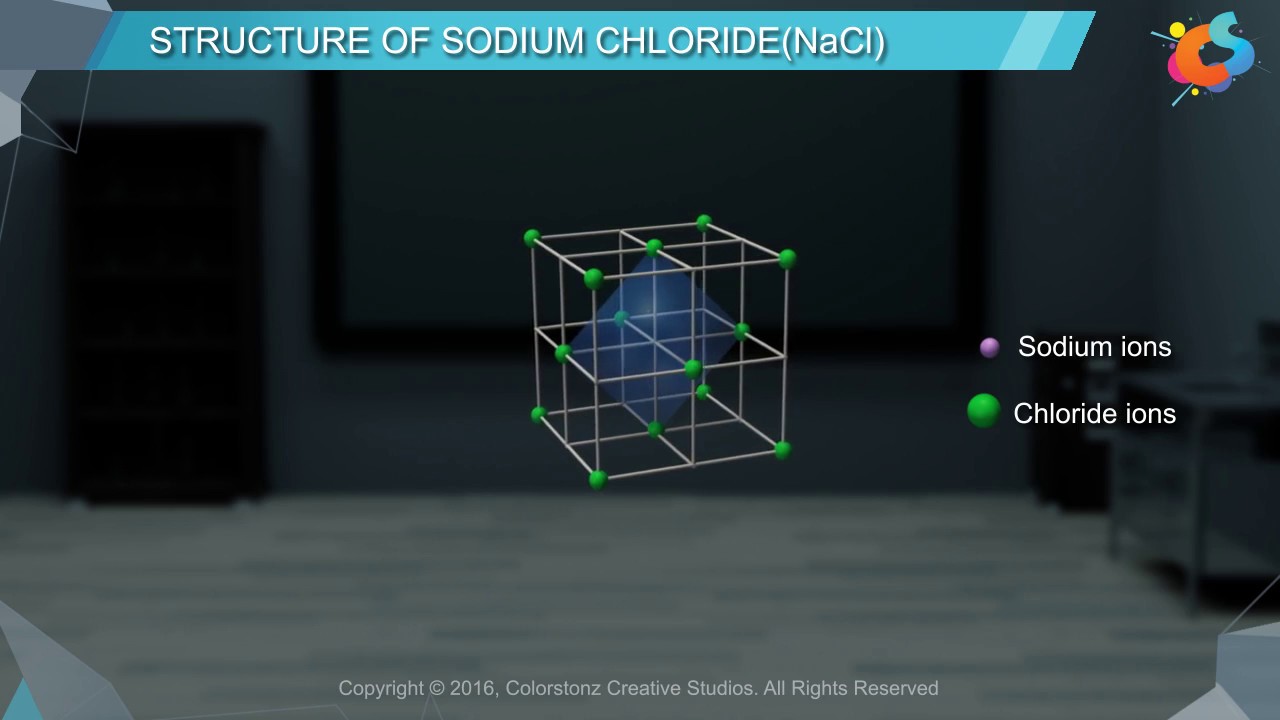
Sodium chloride crystal is made up of sodium and chloride ions. Sodium chloride crystal has a face-centered cubic close packed structure.The chloride ions occupy the corner and face centered positions, the sodium ions occupy the octahedral voids in cubic close structure.The unit cell of NaCl contains 4 chloride ions and 4 sodium ions. The coordination number of sodium chloride is 6.
Structure of Sodium Chloride (NaCl)
Structure of NaCl (Sodium chloride)
NaCl (sodium chloride) crystalline (3D) structure animation
NaCl Crystal lattice | How to draw NaCl Crystal Structure
How To Draw Sodium Chloride (NaCl) Diagram?
Structure of sodium chloride NaCl
How to draw structure of sodium chloride (NaCl)
Chemistry of Salt (NaCl)
Acids, Bases, and Salts Explained | Chemistry for Beginners | Daily Uses Example | NikSuu
Draw the Lewis Structure of NaCl (sodium chloride)
NaCl crystal structure
Sodium Chloride (NaCl) Crystal Animation
Structure of NaCl | Sodium Chloride | Chemistry | iken | ikenApp | ikenEdu
Molecular Structure of Sodium Chloride| BIOCHEMISTRY
Bohr-Rutherford Diagram of NaCl (sodium chloride, table salt)
Sodium Chloride (NaCl) Structure System Explained
Type of Bond for Sodium chloride (NaCl)
how to draw NaCl crystal lattice diagram easily
Discuss the crystal Structure of NaCl. | Solid State | Physical Chemistry
How to Draw the Lewis Dot Structure for NaCl: Sodium chloride
How to make 3D structure of “ NACL”
NaCl Crystal Structure #tricks #shorts #youtubeshorts #chemistry #viral #neet #shortvideo
Ch#6 | Lec#4 | Structure of NaCl | Unit cell of NaCl and Calculation #SodiumChloride #chemistry 11
How To Draw Sodium Chloride Crystal Lattice - Chemistry Animation
Комментарии
 0:02:48
0:02:48
 0:02:10
0:02:10
 0:00:26
0:00:26
 0:03:33
0:03:33
 0:06:19
0:06:19
 0:01:30
0:01:30
 0:02:30
0:02:30
 0:06:23
0:06:23
 0:14:27
0:14:27
 0:02:37
0:02:37
 0:05:06
0:05:06
 0:02:52
0:02:52
 0:02:04
0:02:04
 0:11:32
0:11:32
 0:05:41
0:05:41
 0:18:50
0:18:50
 0:02:21
0:02:21
 0:00:57
0:00:57
 0:02:10
0:02:10
 0:02:07
0:02:07
 0:10:10
0:10:10
 0:00:56
0:00:56
 0:23:48
0:23:48
 0:00:57
0:00:57