filmov
tv
Electronic Configuration of Iron 29- Filling electrons in Iron (Fe) Shells , Sub shells and Orbitals

Показать описание
LIKE, SHARE, COMMENT & SUBSCRIBE "DIGITAL KEMISTRY"
CLICK CHANNEL LINK:
Iron Electronic Configuration - Filling of electrons in Iron (Fe) Shellwise , Sub shellwise and in Orbitals according to Auf bau principle, pauli exclusive principle and hund's rule - Atomic structure class 9 - 10 & class 11 Chemistry .
Follow me on:
For more Chemistry videos:
#digitalkemistry
#chemistryanimation
#ChemistryTipsAndTricksByDigitalKemistry
#onlinechemistryyoutube
#chemistryclass11
#digital
#kemistry
#toptrendingvideos
Digital Kemistry Super Animated Chemistry videos in Urdu, Hindi & English.Also Musical Chemistry videos on my Channel.
Video Links are as follow:
Arrhenius Concept of acid and base:
Bronsted-Lowery concept of acid and base:
Buffer Solution:
Crystalline solid vs Amorphous solid :
CLASSIFICATION OF CRYSTALLINE SOLIDS - SOLID STATES
PROPERTIES OF LIQUIDS: STATE OF MATTER
Kinetic Molecular Theory Of Gases Animation
CLICK CHANNEL LINK:
Iron Electronic Configuration - Filling of electrons in Iron (Fe) Shellwise , Sub shellwise and in Orbitals according to Auf bau principle, pauli exclusive principle and hund's rule - Atomic structure class 9 - 10 & class 11 Chemistry .
Follow me on:
For more Chemistry videos:
#digitalkemistry
#chemistryanimation
#ChemistryTipsAndTricksByDigitalKemistry
#onlinechemistryyoutube
#chemistryclass11
#digital
#kemistry
#toptrendingvideos
Digital Kemistry Super Animated Chemistry videos in Urdu, Hindi & English.Also Musical Chemistry videos on my Channel.
Video Links are as follow:
Arrhenius Concept of acid and base:
Bronsted-Lowery concept of acid and base:
Buffer Solution:
Crystalline solid vs Amorphous solid :
CLASSIFICATION OF CRYSTALLINE SOLIDS - SOLID STATES
PROPERTIES OF LIQUIDS: STATE OF MATTER
Kinetic Molecular Theory Of Gases Animation
Electronic Configuration of Iron 29- Filling electrons in Iron (Fe) Shells , Sub shells and Orbitals
Electron Configuration for Iron (Fe)😉 IN 40 SECONDS!
Electron configuration of Iron II Easy & Quick
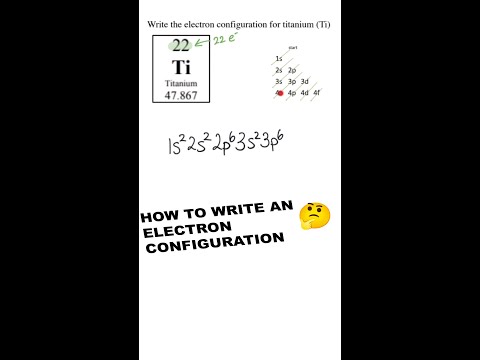
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions)
Electron Configuration - Basic introduction
A step-by-step description of how to write the electron configuration for Iron (Fe).
O Level Chemistry past papers 5070/P22/O/N/23
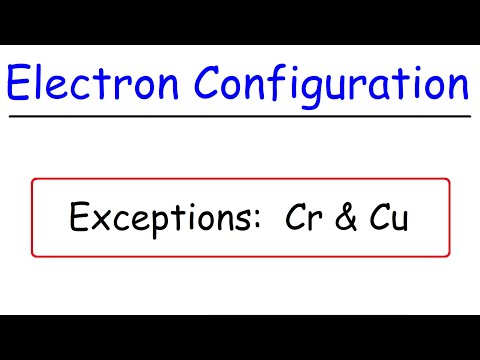
Electron Configuration Exceptions - Chromium (Cr) & Copper (Cu)
Electron Configurations: Transition Metal, Iron
science ka important GK electronic vinyas kaise nikale varal short##
Atomic Structure and Electron Configurations 51: Details of Iron 3+ Ion
Electron Configurations & Diagrams: Fe | AP Chemistry
Write the full electron configuration of iron,
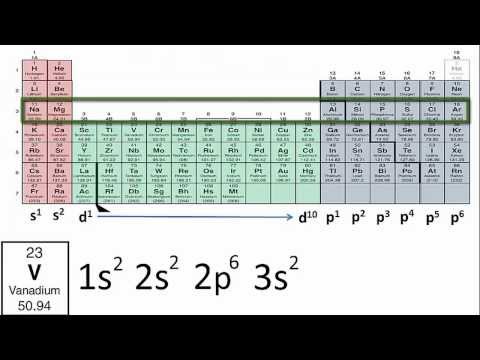
Writing Electron Configurations Using Only the Periodic Table
Iron (Fe) Electron Configuration
Electron Configuration of Iron Fe Lesson
Valence Electrons for Fe (Iron)
The electronic configuration of Cu (atomic number 29) is
Tricks to learn Electronic Configuration #chemistry #class12 #easyway #subscribers #youtuber #viral
How to Write the Atomic Orbital Diagram for Iron (Fe)
Iron 55 electrons
Finding Protons, Electron, Neutrons | Chemistry Class 9 / 10 Science | YouTube Shorts by JP Sir
How to Find Electronic Configuration & Valency of Any Elements | JR Tutorials |
Комментарии
 0:02:39
0:02:39
 0:00:45
0:00:45
 0:01:44
0:01:44
 0:01:00
0:01:00
 0:03:38
0:03:38
 0:10:19
0:10:19
 0:02:56
0:02:56
 1:11:39
1:11:39
 0:05:57
0:05:57
 0:00:56
0:00:56
 0:00:05
0:00:05
 0:05:11
0:05:11
 0:02:55
0:02:55
 0:07:52
0:07:52
 0:04:52
0:04:52
 0:01:23
0:01:23
 0:05:01
0:05:01
 0:03:10
0:03:10
 0:00:36
0:00:36
 0:00:29
0:00:29
 0:02:21
0:02:21
 0:00:52
0:00:52
 0:00:26
0:00:26
 0:01:01
0:01:01