filmov
tv
milliequivalent per liter

Показать описание
(mEq/L)
A unit of measurement used for concentrations of solutions of substances with electrolytes.
(Comparison 1)
• mEq/L: One-thousandth of an equivalent of a chemical per liter.
• mmol/L: One-thousandth of a gram molecule per liter.
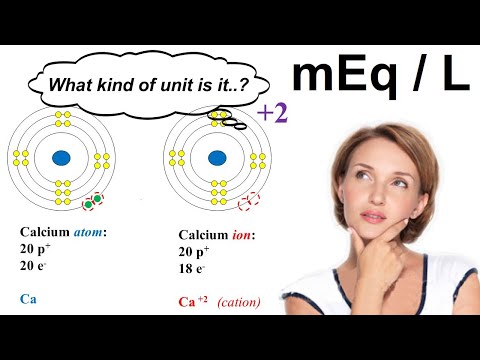
For example, for concentration of sodium, 1 mEq/L is the same as 1 mmol/L, because the number of equivalents of a sodium ion (Na+) is one. On the other hand, for concentration of calcium, 2 mEq/L is the same as 1 mmol/L, because the number of equivalents of a calcium ion (Ca2+) is two.
(Comparison 2)
• eq/L: Equivalent per liter.
• N: Normality or quivalent concentration.
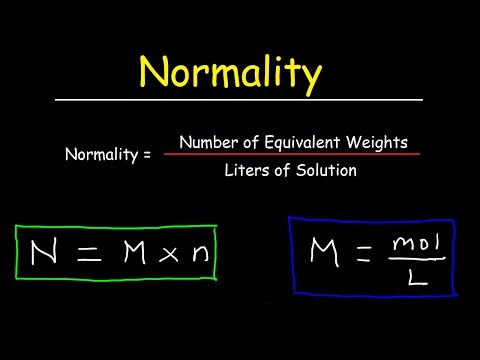
The equivalent concentration of a solution (N) is defined as the molar concentration (mol/L) divided by an equivalence factor.
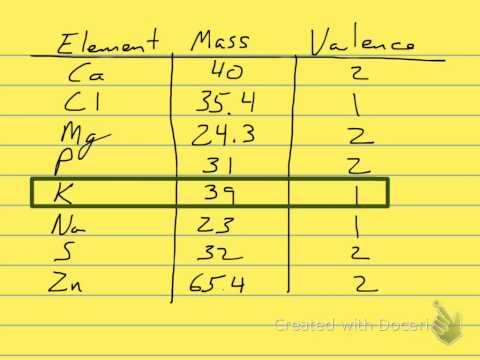
For example, for the concentration of sodium solution, 1 N equals to 1 mol/L, because the equivalence factor is 1 (Na+). On the other hand, for the concentration of calcium solution, 1 N equals to 2 mol/L, because the equivalence factor is 2 (Ca2+).
A unit of measurement used for concentrations of solutions of substances with electrolytes.
(Comparison 1)
• mEq/L: One-thousandth of an equivalent of a chemical per liter.
• mmol/L: One-thousandth of a gram molecule per liter.
For example, for concentration of sodium, 1 mEq/L is the same as 1 mmol/L, because the number of equivalents of a sodium ion (Na+) is one. On the other hand, for concentration of calcium, 2 mEq/L is the same as 1 mmol/L, because the number of equivalents of a calcium ion (Ca2+) is two.
(Comparison 2)
• eq/L: Equivalent per liter.
• N: Normality or quivalent concentration.
The equivalent concentration of a solution (N) is defined as the molar concentration (mol/L) divided by an equivalence factor.
For example, for the concentration of sodium solution, 1 N equals to 1 mol/L, because the equivalence factor is 1 (Na+). On the other hand, for the concentration of calcium solution, 1 N equals to 2 mol/L, because the equivalence factor is 2 (Ca2+).
 0:02:00
0:02:00
 0:03:41
0:03:41
 0:04:01
0:04:01
 0:11:47
0:11:47
 0:11:23
0:11:23
 0:01:24
0:01:24
 0:02:21
0:02:21
 0:06:59
0:06:59
 0:01:48
0:01:48
 0:34:46
0:34:46
 0:16:49
0:16:49
 0:05:09
0:05:09
 0:05:15
0:05:15
 0:00:27
0:00:27
 0:04:34
0:04:34
 0:01:43
0:01:43
 0:01:08
0:01:08
 0:00:47
0:00:47
 0:04:12
0:04:12
 0:00:56
0:00:56
 0:15:49
0:15:49
 0:05:32
0:05:32
 0:15:26
0:15:26
 0:08:55
0:08:55