filmov
tv
Combined Gas Equation
Показать описание
Combined Gas Equation
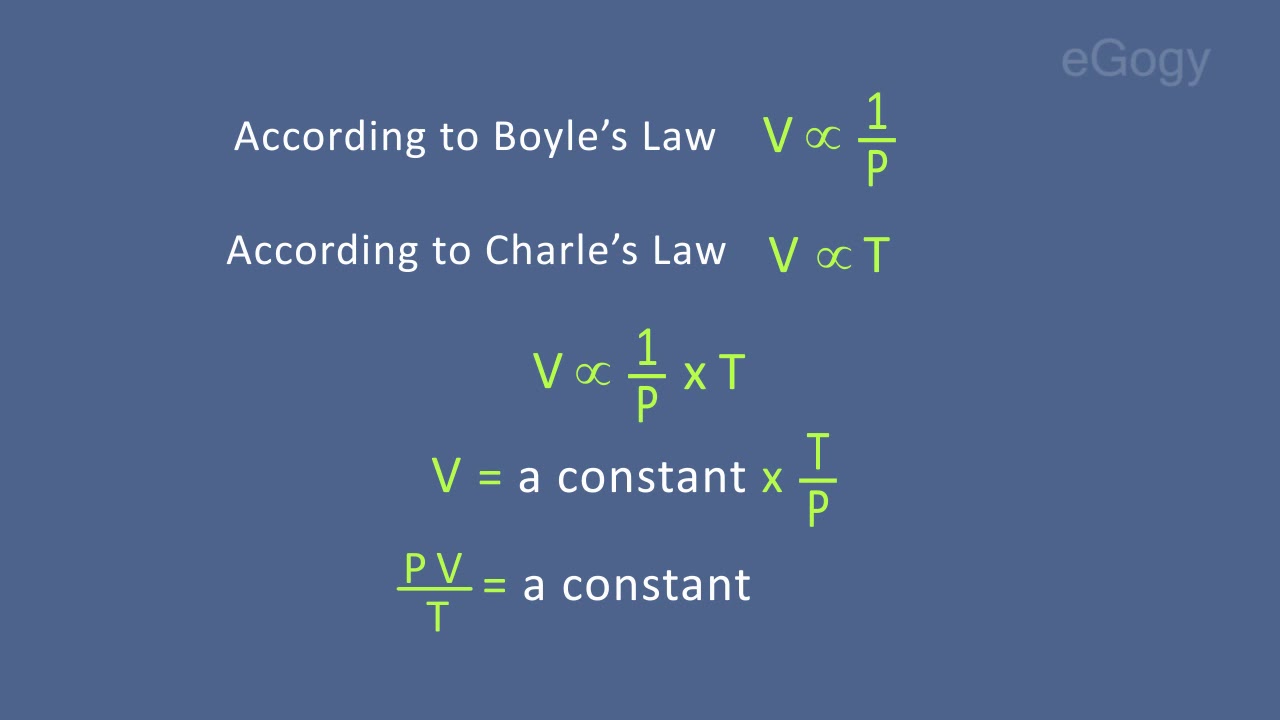
The equation which combines both Boyle’s law and Charle’s law is called combined gas equation.
According to Boyle’s Law V α 1/P
According to Charle’s Law V α T
By combining these two laws we get,
V α 1/P x T
V = a constant x 1/P x T
.·. PV/T = a constant.
P1 V1 / T1 = P2 V2 / T2.
If the pressure, volume and temperature of a fixed mass of gas are changed from P1, V1 and T1 to P2, V2 and T2 respectively
This equation is known as Combined gas equation.
The equation which combines both Boyle’s law and Charle’s law is called combined gas equation.
According to Boyle’s Law V α 1/P
According to Charle’s Law V α T
By combining these two laws we get,
V α 1/P x T
V = a constant x 1/P x T
.·. PV/T = a constant.
P1 V1 / T1 = P2 V2 / T2.
If the pressure, volume and temperature of a fixed mass of gas are changed from P1, V1 and T1 to P2, V2 and T2 respectively
This equation is known as Combined gas equation.
Combined Gas Equation
Combined Gas Law Problems
Combined Gas Law: Explanation, Practice, and Examples
Combined Gas Law Explained!
When to use the Combined Gas Law or the Ideal Gas Law
Kinetic Molecular Theory and the Ideal Gas Laws
Gas Law Formulas and Equations - College Chemistry Study Guide
The Ideal Gas Law: Crash Course Chemistry #12
Class-11 Physics- Thermodynamics-04- Ideal Gas Equation, Gas laws, KTG JEE/NEET - MG Sir
Combined Gas Law (P1V1/T1 = P2V2/T2) Examples, Practice Problems, Calculations, Equation
Combined Gas Law
Ideal Gas law vs Combined Gas Law....Which One?
Derivation Of The Combined Gas Law Equation | Amoeba Classes
Gases 7 - Combined Gas Law
Grade 11 - Gas Laws | Combined Gas Laws | Mlungisi Nkosi
Combined gas law|Characteristic gas equation|GTU|PV=mRT|Derive PV=RT|BME|Equation of state
Combined Gas Law - Pressure, Volume and Temperature - Straight Science
Gas Laws-Boyle's-Charles's-Gay Lussac's
Combined Gas Law | Chemistry Homework in 3 MINUTES
Combined Gas Law
Combined Gas Law😀🎈 #chemistry #science #shorts #short #homework
Combined Gas Law Formula (Chemistry)
How to Use Each Gas Law | Study Chemistry With Us
Ideal Gas Law | General Gas Equation | Chemistry
Комментарии
 0:01:05
0:01:05
 0:12:06
0:12:06
 0:05:07
0:05:07
 0:01:00
0:01:00
 0:03:59
0:03:59
 0:05:11
0:05:11
 0:19:24
0:19:24
 0:09:03
0:09:03
 1:12:10
1:12:10
 0:07:55
0:07:55
 0:02:43
0:02:43
 0:04:27
0:04:27
 0:04:38
0:04:38
 0:04:30
0:04:30
 0:18:12
0:18:12
 0:07:54
0:07:54
 0:09:25
0:09:25
 0:02:34
0:02:34
 0:03:12
0:03:12
 0:10:35
0:10:35
 0:00:51
0:00:51
 0:11:40
0:11:40
 0:26:34
0:26:34
 0:06:59
0:06:59