filmov
tv
Group 7 Chemical Properties: Diatomic Molecules (Halogens) - GCSE Chemistry | kayscience.com
Показать описание
In this video you will learn all the science for this topic to get a grade 9 or A* in your science exams!
In this video, you will learn this model answer to achieve 100% in any exam question:
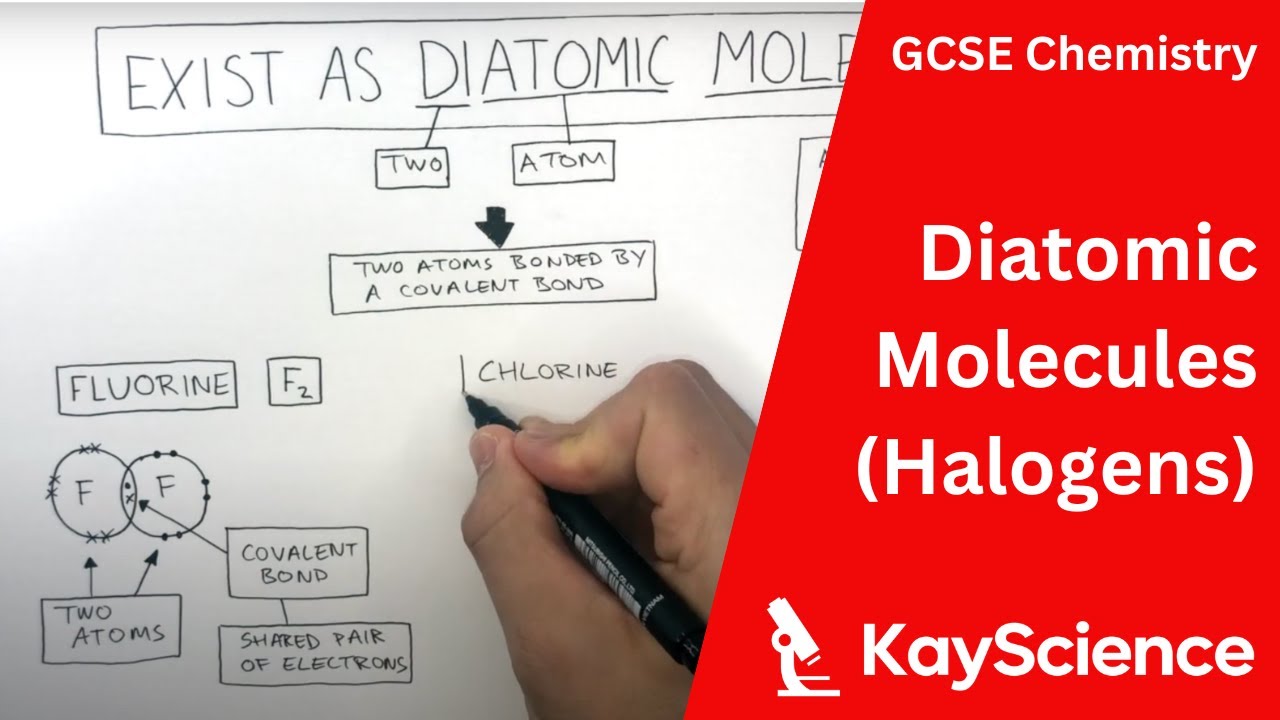
Group 7 elements are known as halogens. They are in group 7 so have 7 electrons in their outer shell. They share their outer electron with another atom of the same element to achieve a full outer shell. Halogens can form diatomic molecules which are two atoms covalently bonded together. The covalent bonds between the atoms are strong, and there are weak intermolecular forces between the molecules.
In this video, you will learn this model answer to achieve 100% in any exam question:
Group 7 elements are known as halogens. They are in group 7 so have 7 electrons in their outer shell. They share their outer electron with another atom of the same element to achieve a full outer shell. Halogens can form diatomic molecules which are two atoms covalently bonded together. The covalent bonds between the atoms are strong, and there are weak intermolecular forces between the molecules.
 0:05:52
0:05:52
 0:04:49
0:04:49
 0:11:18
0:11:18
 0:01:14
0:01:14
 0:05:15
0:05:15
 0:12:34
0:12:34
 0:34:54
0:34:54
 0:05:15
0:05:15
 0:06:16
0:06:16
 0:09:12
0:09:12
 0:16:02
0:16:02
 0:21:16
0:21:16
 0:07:06
0:07:06
 0:03:33
0:03:33
 0:12:23
0:12:23
 0:03:28
0:03:28
 0:11:28
0:11:28
 0:30:55
0:30:55
 0:06:53
0:06:53
 0:06:28
0:06:28
 0:00:50
0:00:50
 0:04:36
0:04:36
 0:00:59
0:00:59
 0:06:34
0:06:34