filmov
tv
How is Nuclear Stability Related to the Band of Stability and the Neutron to Proton Ratio

Показать описание
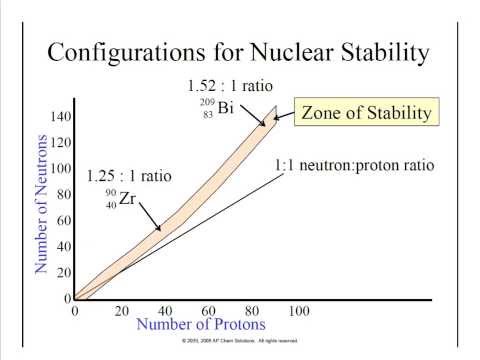
An isotope's stability depends on where its neutron-to-proton ratio falls on the band of stability and the existence of certain magic numbers.
What are stable isotopes? Stable isotopes are those that do not undergo radioactive decay. The neutron-to-proton ratio of these isotopes fall on the band of stability.
Isotopes whose neutron-to-proton ratio do not fall on the band of stability, on the other hand, are unstable, and therefore radioactive.
This is generally the case when an isotope's atomic number, number of protons, is greater than 83. Large isotopes tend to decay by alpha radiation, because massive alpha particles are given off.
Lighter isotopes whose neutron-to-proton ratios do not fall on the band of stability will also be radioactive. If an isotope's neutron-to-proton ratio falls to the left of the band of stability, meaning that it has too many neutrons for its number of protons, it will tend to undergo beta decay, in which a beta particle is emitted, decreasing the number of neutrons while increasing the number of protons. If an isotope's neutron-to-proton ratio falls to the right of the band of stability, meaning that it has too few neutrons for its number of protons, it will tend to undergo positron emission, in which a positron is emitted, increasing the number of neutrons while decreasing the number of protons.
In this chemistry lesson, you will learn:
why some isotopes are radioactive
why isotopes decay
how to determine the neutron-to-proton ratio of an isotope
how to identify the most stable isotope by comparing its neutron-to-proton ratio to the band of stability
which type of decay a radioactive isotope will undergo, depending on its neutron-to-proton ratio and where it falls on the band of stability
how magic numbers of protons and neutrons help predict the stability of an isotope
The 3-step checklist for predicting the stability of an isotope
And solve these sample problems:
Determine the neutron-to-proton ratio of C-12, C-13 and C-14, and identify the most stable isotope.
Is Mn-54 Stable?
Write the equation for the probable mode of decay of Aluminum-28.
You can learn more about the neutron-to-proton ratio and the band of stability definition by visiting:
To learn more about alpha, beta and gamma decay, watch my video here:
This video is created by an experienced Chemistry teacher for high school students who are looking to learn more about nuclear stability, the neutron-to-proton ratio, and the band of stability.
We encourage you to share this video using this web address:
To subscribe to our channel for more useful videos related to high school Chemistry simply visit:
how is nuclear stability related to the neutron-proton ratio
neutron-to-proton ratio
band of stability definition
what are stable isotopes
chemistry lessons
how is nuclear stability related to the neutron-proton ratio
band of stability definition
neutron-to-proton ratio
what are stable isotopes
Комментарии
 0:10:48
0:10:48
 0:05:10
0:05:10
 0:04:31
0:04:31
 0:14:12
0:14:12
 0:05:22
0:05:22
 0:07:27
0:07:27
 0:02:48
0:02:48
 0:03:04
0:03:04
 0:29:19
0:29:19
 0:08:34
0:08:34
 0:04:41
0:04:41
 0:06:39
0:06:39
 0:13:57
0:13:57
 0:01:57
0:01:57
 0:05:07
0:05:07
 0:15:59
0:15:59
 0:05:19
0:05:19
 0:44:59
0:44:59
 0:08:36
0:08:36
 0:11:18
0:11:18
 0:03:21
0:03:21
 0:04:09
0:04:09
 0:11:32
0:11:32
 0:09:35
0:09:35