filmov
tv
Calculate Equilibrium Partial Pressure

Показать описание
For the reaction CaCO3(s) ↔ CaO(s)+CO2(g) at 25°C, ΔGΘ=130,4 kJ/mol,
ΔHΘ=178,3 kJ/mol and ΔHΘ is not a function of temperature.
Calculate the equilibrium pressure of CO2 at 298.15K and 1100.15K.
ΔHΘ=178,3 kJ/mol and ΔHΘ is not a function of temperature.
Calculate the equilibrium pressure of CO2 at 298.15K and 1100.15K.
Worked example: Using the reaction quotient to find equilibrium partial pressures | Khan Academy
Worked example: Calculating an equilibrium constant from initial and equilibrium pressures
Calculate Equilibrium Partial Pressure
Partial Pressure Equilibrium Constant
Calculate Equilibrium Partial Pressure
Equilibrium Partial Pressures | General Chemistry II | 7.4
Partial pressure - Gas equilibria
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
Crack JEE 2025 | JEE Mantra Series 2024 | Chemistry Recap Video Lecture - 7 | JEE Main Preparation
Finding Equilibrium Partial Pressures-Practice Problems (Part 1)
⚗️ Finding Equilibrium Partial Pressures Using an ICE Table
How to find the equilibrium partial pressures of a reaction using an ICE table
Calculate equilibrium pressure from [ICE Table] 2018
Calculate partial pressure of B at equilibrium in the following equilibrium `A(s) if
Chapter 31 HW 22 calculation of equil partial pressures (part IV)
Equilibrium Pressures
Find the equilibrium partial pressures from initial pressures
equilibrium partial pressure problem solved
Calculating Equilibrium Constants from Partial Pressures
Calculate partial pressure of B at equilibrium in the following equilibrium A(s) ⇌ B(g)+2 C(......
Worked examples: Calculating equilibrium constants | Equilibrium | AP Chemistry | Khan Academy
Example of Calculating Equilibrium Concentrations when given only K and initial pressure
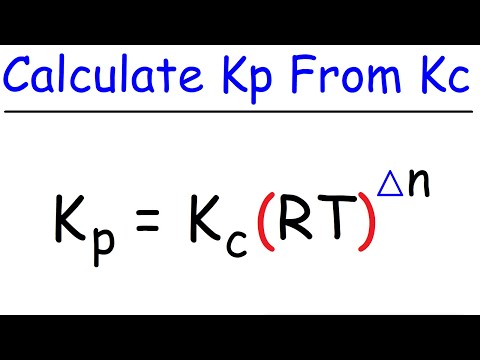
How To Calculate Kp From Kc - Chemical Equilibrium
Lesson 5 Kp & its applications|calculating equilibrium constant using partial pressures & vi...
Комментарии
 0:06:31
0:06:31
 0:04:51
0:04:51
 0:14:53
0:14:53
 0:02:15
0:02:15
 0:14:53
0:14:53
 0:05:56
0:05:56
 0:08:42
0:08:42
 0:53:22
0:53:22
 0:42:44
0:42:44
 0:12:07
0:12:07
 0:08:25
0:08:25
 0:05:49
0:05:49
 0:03:59
0:03:59
 0:02:32
0:02:32
 0:13:56
0:13:56
 0:05:35
0:05:35
 0:03:59
0:03:59
 0:02:03
0:02:03
 0:05:33
0:05:33
 0:01:53
0:01:53
 0:08:12
0:08:12
 0:06:14
0:06:14
 0:10:51
0:10:51
 0:04:32
0:04:32