filmov
tv
Structure of the Atom Full Chapter | NCERT Explanation | CBSE Class 9 Chemistry | Shubham Pathak

Показать описание
This is your educator SHUBHAM PATHAK. This new channel is solely for Class 9 students where I will be teaching you :
A) Class 9 SST
B) Class 9 Biology
C) Class 9 Chemistry
D) Class 10 Physics
E) Tips and Tricks for your exams, and MUCH MORE!
You might also like :
If you have any session to request, or you just want to talk to me, please connect :
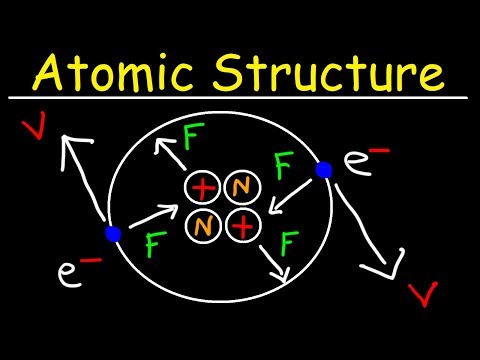
About this Chapter : This is a very interesting chapter of your class 9 science which teaches us about atoms, sub atomic particles, and structure of atom.
In this Video :
A) Class 9 Science
B) Class 9 Chemistry
C) Class 9 Structure of the Atom full chapter
D) Cathode Ray Experiment by Thompson
E) Canal Ray Experiment by Goldstein
F) Structure of the Atom
G) JJ Thompson model of atom
H) Rutherford Model of atom
I) Alpha Ray experiment Rutherford
J) Bohr model of atom
K) Structure of Atom NCERT Explanation
L) Istopes
M) Isobars
N) Atomic Number and Mass number
Thank you for reading this.
#structureoftheatomfullchapter #class9scienceterm2 #shubhampathak
A) Class 9 SST
B) Class 9 Biology
C) Class 9 Chemistry
D) Class 10 Physics
E) Tips and Tricks for your exams, and MUCH MORE!
You might also like :
If you have any session to request, or you just want to talk to me, please connect :
About this Chapter : This is a very interesting chapter of your class 9 science which teaches us about atoms, sub atomic particles, and structure of atom.
In this Video :
A) Class 9 Science
B) Class 9 Chemistry
C) Class 9 Structure of the Atom full chapter
D) Cathode Ray Experiment by Thompson
E) Canal Ray Experiment by Goldstein
F) Structure of the Atom
G) JJ Thompson model of atom
H) Rutherford Model of atom
I) Alpha Ray experiment Rutherford
J) Bohr model of atom
K) Structure of Atom NCERT Explanation
L) Istopes
M) Isobars
N) Atomic Number and Mass number
Thank you for reading this.
#structureoftheatomfullchapter #class9scienceterm2 #shubhampathak
Комментарии
 0:30:31
0:30:31
 1:01:07
1:01:07
 0:02:20
0:02:20
 0:11:45
0:11:45
 0:01:44
0:01:44
 1:57:06
1:57:06
 0:09:49
0:09:49
 0:00:24
0:00:24
 0:10:36
0:10:36
 0:00:12
0:00:12
 0:02:02
0:02:02
 1:04:43
1:04:43
 1:29:48
1:29:48
 5:44:28
5:44:28
 3:11:55
3:11:55
 0:59:50
0:59:50
 0:37:06
0:37:06
 7:19:15
7:19:15
 0:04:06
0:04:06
 2:32:00
2:32:00
 5:13:38
5:13:38
 0:15:41
0:15:41
 0:08:42
0:08:42
 0:09:42
0:09:42