filmov
tv
Periodic Table (MADE EASY 😍🥳) | Electron configuration: s, p, d, f

Показать описание
The periodic table is one the most fundamental concepts in all of chemistry. We begin with a discussion of the mass number (m) and the atomic number (z), which are used to identify atoms on the periodic table.
The electron orbital clouds of each atom influence the periodic table's structure, and subsequently, the location of each atom on the periodic table. There is an order for how the s, p, d, and f orbitals are filled. Finally, we take a look at how to write the long electron notation for each atom.
Instructor: Dr. David Carlson, MD
How to Read the Periodic Table
How To Memorize The Periodic Table - Easiest Way Possible (Video 1)
Learn Periodic Table in 5 minutes || English and Urdu both languages
The Periodic Table: Crash Course Chemistry #4
Learn the Basics of the Periodic Table!
Periodic Table
The Periodic Table Explained
How To Memorize The Periodic Table - Easiest Way Possible to Remember Elements!
#periodictable #children #education #chemistry #facts #life #family #nature #animation #funny
GCSE Chemistry - Modern Periodic Table #9
The Periodic Table Song (2018 Update!) | SCIENCE SONGS
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Periodic Table Explained! #chemistry #science
Trick to Learn Periodic Table #shorts
Perfecting the Periodic Table
GENERAL CHEMISTRY explained in 19 Minutes
Modern Periodic Table
The Periodic Table Song.. ( credit goes to the rightful owner)
Periodic Table Explained: Introduction
How To Memorize Periodic Table? Super Easy Trick
How To Memorize The Periodic Table Through Practice!
PERIODIC TABLE(elements song)
Awesome Trick to learn periodic table in 5 min
How To Memorize The Periodic Table - Easiest Way Possible (Video 2)
Комментарии
 0:03:36
0:03:36
 0:05:14
0:05:14
 0:06:03
0:06:03
 0:11:22
0:11:22
 0:07:08
0:07:08
 0:24:09
0:24:09
 0:03:07
0:03:07
 0:07:17
0:07:17
 0:00:58
0:00:58
 0:05:36
0:05:36
 0:03:05
0:03:05
 0:07:53
0:07:53
 0:00:57
0:00:57
 0:00:56
0:00:56
 0:04:31
0:04:31
 0:18:49
0:18:49
 0:18:53
0:18:53
 0:00:31
0:00:31
 0:14:14
0:14:14
 0:08:15
0:08:15
 0:25:36
0:25:36
 0:00:55
0:00:55
 0:05:15
0:05:15
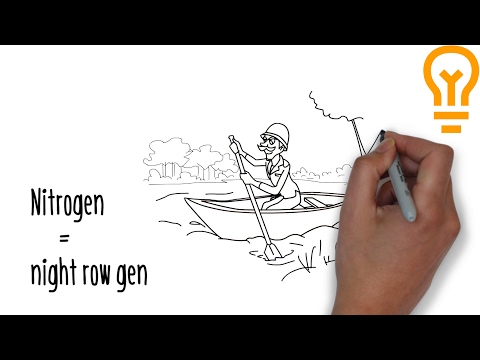 0:05:26
0:05:26