filmov
tv
Cathode Ray Tube Experiment and Charge To Mass Ratio of an Electron
Показать описание
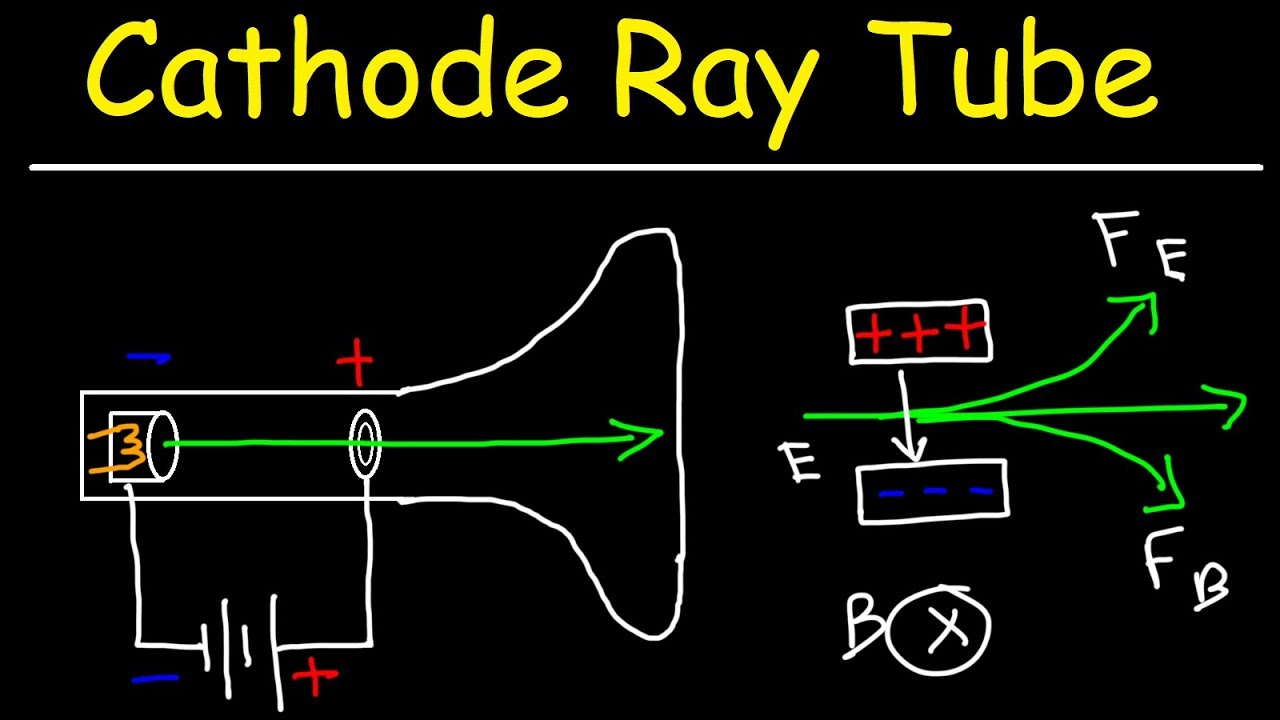
This chemistry and physics video tutorial provides a basic introduction into the cathode ray tube experiment. JJ Thompson used this experiment to conclude that all atoms contain negatively charged particles known as electrons. Using this experiment, he was able to calculate the charge to mass ratio of an electron.
Chemistry - Basic Introduction:
Scientific Notation Review:
Significant Figures Review:
Unit Conversion Problems:
Accuracy and Precision:
Density Practice Problems:
________________________________
Pure Substances & Mixtures:
Homogeneous & Heterogeneous Mixtures:
Physical and Chemical Changes:
Solids, Liquids, Gases, & Plasma:
Physical Vs Chemical Properties:
__________________________________
Law of Conservation of Mass:
Law of Definite Proportions:
Law of Multiple Proportions:
Rutherford's Gold Foil Experiment:
Cathode Ray Tube Experiment:
_________________________________
Atoms - Basic Introduction:
Cations and Anions Explained:
Diatomic Elements & Molecules:
Elements, Atoms, & Molecules:
Protons, Neutrons, & Electrons:
_______________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry - Basic Introduction:
Scientific Notation Review:
Significant Figures Review:
Unit Conversion Problems:
Accuracy and Precision:
Density Practice Problems:
________________________________
Pure Substances & Mixtures:
Homogeneous & Heterogeneous Mixtures:
Physical and Chemical Changes:
Solids, Liquids, Gases, & Plasma:
Physical Vs Chemical Properties:
__________________________________
Law of Conservation of Mass:
Law of Definite Proportions:
Law of Multiple Proportions:
Rutherford's Gold Foil Experiment:
Cathode Ray Tube Experiment:
_________________________________
Atoms - Basic Introduction:
Cations and Anions Explained:
Diatomic Elements & Molecules:
Elements, Atoms, & Molecules:
Protons, Neutrons, & Electrons:
_______________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:18:08
0:18:08
 0:11:08
0:11:08
 0:01:58
0:01:58
 0:01:52
0:01:52
 0:03:13
0:03:13
 0:10:38
0:10:38
 0:01:11
0:01:11
 0:13:29
0:13:29
 0:22:55
0:22:55
 0:03:30
0:03:30
 0:03:20
0:03:20
 0:00:57
0:00:57
 0:00:44
0:00:44
 0:00:53
0:00:53
 0:03:18
0:03:18
 0:09:38
0:09:38
 0:02:36
0:02:36
 0:02:32
0:02:32
 0:04:54
0:04:54
 0:11:56
0:11:56
 0:03:14
0:03:14
 0:07:07
0:07:07
 0:01:48
0:01:48
 0:02:44
0:02:44