filmov
tv
Matter Waves and De Broglie's Hypothesis

Показать описание
Matter Waves and De Broglie's Hypothesis. Mr. Causey explains De Broglie's hypothesis, matter waves and Schrodinger's equation in the development of the wave-mechanical model of the atom.
SUBSCRIBE for more chemistry videos:
ABOUT MR. CAUSEY'S VIDEO ACADEMY
Mr. Causey's Video Academy is an educational video series of short video lessons for chemistry, algebra and physics. You can get lessons on a variety of topics or homework helpers that show you how to solve certain problems. There are over 120 videos to choose from.
CONTACT ME:
FOLLOW ME:
RESOURCES:
Polyatomic Ion Cheat Sheet:
Periodic Table:
RELATED VIDEOS:
Planetary Model:
quantum mechanical model:
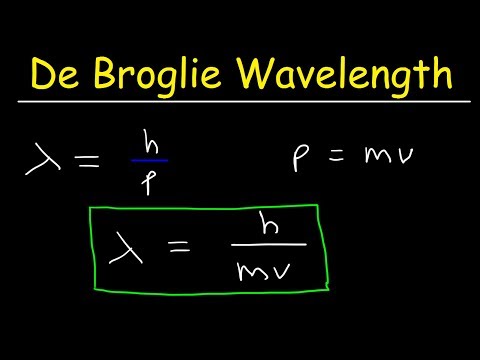
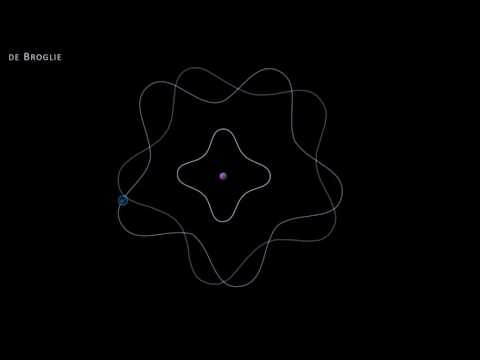
Mr. Causey explains De Broglie's hypothesis, matter waves and Schrodinger's equation in the development of the wave-mechanical model of the atom. Louis de Broglie proposes the idea that if light can have particle properties then particles should have wave properties. He based his hypothesis on the symmetry of nature and Einstein's formulas from the explanation of the photoelectric effect. In effort to gain support for his idea de Broglie sent his work to Einstein who readily accepted this new hypothesis. However, de Broglie's hypothesis was confirmed until 1927 by Germer and Davison and independently by G.P. Thomson.
Erwin Schrodinger takes the De Broglie Hypothesis and combines it with the Planetary Model to create the Wave Mechanical Model. Schrodinger suggest that the matter wave is a three dimensional standing wave and develops the Schrodinger equation to support it mathematically,
SUBSCRIBE for more chemistry videos:
ABOUT MR. CAUSEY'S VIDEO ACADEMY
Mr. Causey's Video Academy is an educational video series of short video lessons for chemistry, algebra and physics. You can get lessons on a variety of topics or homework helpers that show you how to solve certain problems. There are over 120 videos to choose from.
CONTACT ME:
FOLLOW ME:
RESOURCES:
Polyatomic Ion Cheat Sheet:
Periodic Table:
RELATED VIDEOS:
Planetary Model:
quantum mechanical model:
Mr. Causey explains De Broglie's hypothesis, matter waves and Schrodinger's equation in the development of the wave-mechanical model of the atom. Louis de Broglie proposes the idea that if light can have particle properties then particles should have wave properties. He based his hypothesis on the symmetry of nature and Einstein's formulas from the explanation of the photoelectric effect. In effort to gain support for his idea de Broglie sent his work to Einstein who readily accepted this new hypothesis. However, de Broglie's hypothesis was confirmed until 1927 by Germer and Davison and independently by G.P. Thomson.
Erwin Schrodinger takes the De Broglie Hypothesis and combines it with the Planetary Model to create the Wave Mechanical Model. Schrodinger suggest that the matter wave is a three dimensional standing wave and develops the Schrodinger equation to support it mathematically,
Комментарии
 0:11:20
0:11:20
 0:19:00
0:19:00
 0:09:05
0:09:05
 0:11:21
0:11:21
 0:10:37
0:10:37
 0:20:24
0:20:24
 0:08:12
0:08:12
 0:03:32
0:03:32
 1:35:21
1:35:21
 0:08:33
0:08:33
 0:10:26
0:10:26
 0:01:43
0:01:43
 0:16:28
0:16:28
 0:07:01
0:07:01
 0:14:50
0:14:50
 0:11:14
0:11:14
 0:16:45
0:16:45
 0:06:39
0:06:39
 0:11:53
0:11:53
 0:00:58
0:00:58
 0:12:21
0:12:21
 0:07:16
0:07:16
 0:00:48
0:00:48
 0:04:47
0:04:47