filmov
tv
First and second ionization energy | Atomic structure and properties | AP Chemistry | Khan Academy

Показать описание
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
First and second ionization energy | Atomic structure and properties | AP Chemistry | Khan Academy
CHEMISTRY 101: Trends in Ionization Energies
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Ionization Energy - Basic Introduction
Ionization Energy || Types of Ionization Energy || First Second and Third Ionization Energy
How would you distinguish between the first, second, and third ionization energies of an atom?
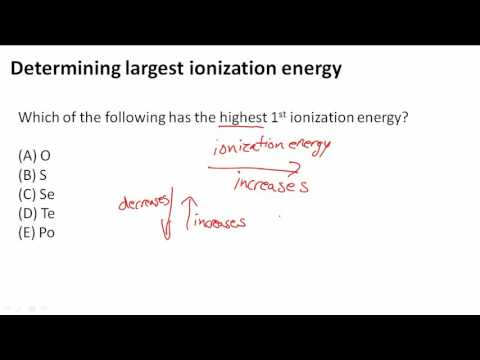
Determining largest ionization energy
First and Second Ionization Energy | Homeworks.ng
Chemistry For JEE-Main 2025 | Classification and Periodicity of Elements | Bilingual
Ionization Energy | Periodic Trends
Ionization Energy Equations
Second Ionization Energy
Practice Problem: Ionization Energy
Worked example: Identifying an element from successive ionization energies | Khan Academy
The electronic configuration having maximum difference in first and second ionization energies i....
IB Chemistry: Ionisation Energy
1st ionization energy higher for N, but 2nd for O #chemistry #shorts
The first and the second ionization enthalpies and the electron gain enthalpy of a few elements
Trends in Ionisation Energies | AS-Level Chemistry
What is ionization energy What is the difference between first ionization energy and second ionizati
CHEMISTRY 101: Periodic Trends for Ionization Energy
11 chap 3 | Periodi c Table 05 | Ionisation Energy | Ionisation Energy IIT Ionisation Potential IIT
2nd Ionization energy is greater than 1st Ionization energy | ch#8 | 9th class chemistry
Ionization energy trends | Periodic table | Chemistry | Khan Academy
Комментарии
 0:07:35
0:07:35
 0:03:23
0:03:23
 0:07:53
0:07:53
 0:32:48
0:32:48
 0:04:17
0:04:17
 0:03:39
0:03:39
 0:01:42
0:01:42
 0:07:35
0:07:35
 0:29:47
0:29:47
 0:10:59
0:10:59
 0:02:56
0:02:56
 0:08:23
0:08:23
 0:07:08
0:07:08
 0:02:31
0:02:31
 0:01:24
0:01:24
 0:10:21
0:10:21
 0:01:00
0:01:00
 0:09:24
0:09:24
 0:15:16
0:15:16
 0:01:55
0:01:55
 0:06:17
0:06:17
 0:51:33
0:51:33
 0:07:27
0:07:27
 0:10:01
0:10:01