filmov
tv
Retrosynthesis 11 - Organic Chemistry
Показать описание
Retrosynthesis in organic chemistry, showcasing partial alkyne reduction, Diels-Alder reaction, and aldehyde homologation.
#organicchemistry #chemistry #orgo #ochem #science #stem #education
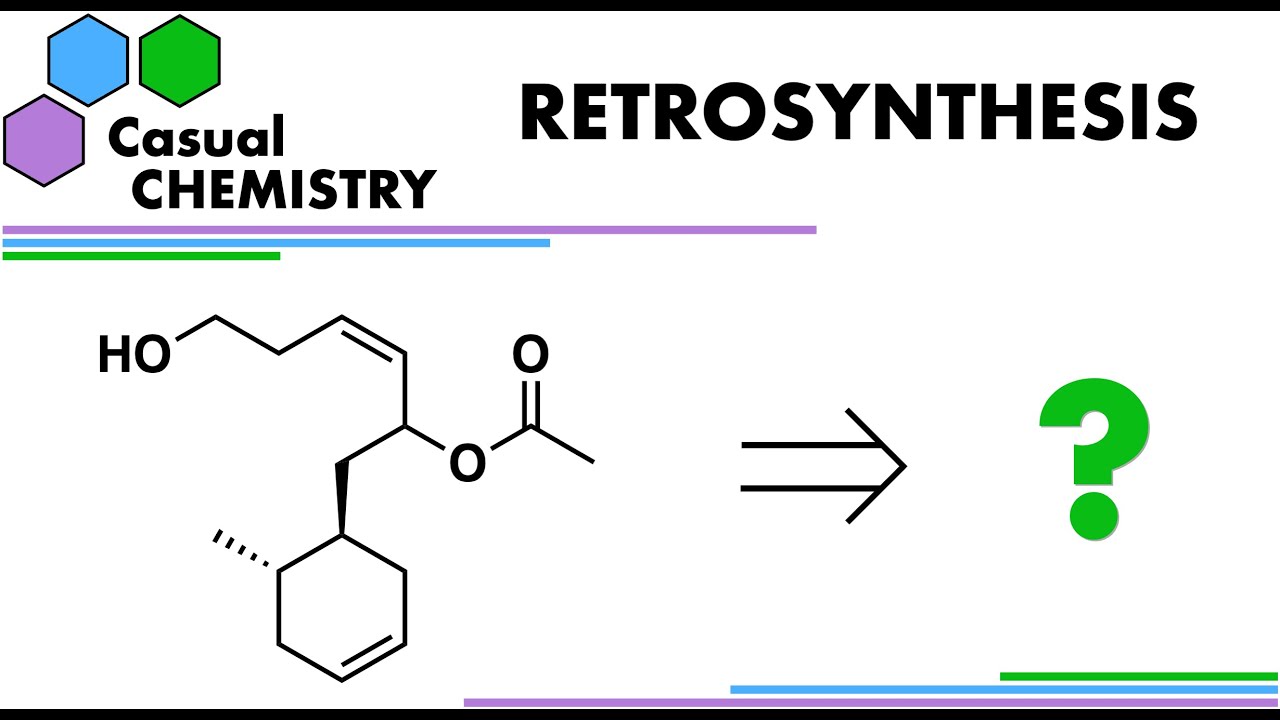
The retrosynthetic analysis of this molecule starts by analysing the functional groups present. The acidic proton on the primary alcohol hydroxyl group will need a protecting group, perhaps as a silyl ether. The cis alkene can be synthesised by partial reduction of an alkyne by Lindlar reduction – a hydrogenation reaction with a poisoned catalyst. The alkyne is a useful intermediate as it can be used as a good nucleophile when an unfunctionalized alkyne is deprotonated. The anion of an alkyne is easy to deprotonate, with a proton of pKa around 25, and is also a good nucleophile as it is not sterically hindered. In this molecule, a hydroxyl protected butynol can be deprotonated and used as a nucleophile for an aldehyde to comprise of the other half of the molecule.
The aldehyde component also contains a cyclohexene motif, which is a classic disconnection pattern for a Diels-Alder reaction. A Diels-Alder reaction is a pericyclic cycloaddition that could be done here with butadiene, but the reaction also requires a dienophile with and electron-withdrawing group directly attached. At first sight, we cannot the aldehyde carbonyl is too far away from the C=C alkene bond, being unconjugated. However, in a retrosynthesis, we could simply disconnect with a homologation reaction. A homologation reaction is one that increases a carbon chain length to the next member of a homologous series in organic chemistry. In this case, we need to add a methylene unit (CH2), and an aldehyde can be homologated by Wittig reaction with a specific ylid. This ylid is methoxymethylenetriphenylphosphine (Ph3P=CH(OMe)), which can be prepared by P-alkylation of triphenylphoshine, and subsequent deprotonation of the phosphonium salt to the ylid by LDA, for example. This reaction forms an enol ether product and this product can be revealed as the homologated aldehyde on further reaction with aqueous acid.
#organicchemistry #chemistry #orgo #ochem #science #stem #education
The retrosynthetic analysis of this molecule starts by analysing the functional groups present. The acidic proton on the primary alcohol hydroxyl group will need a protecting group, perhaps as a silyl ether. The cis alkene can be synthesised by partial reduction of an alkyne by Lindlar reduction – a hydrogenation reaction with a poisoned catalyst. The alkyne is a useful intermediate as it can be used as a good nucleophile when an unfunctionalized alkyne is deprotonated. The anion of an alkyne is easy to deprotonate, with a proton of pKa around 25, and is also a good nucleophile as it is not sterically hindered. In this molecule, a hydroxyl protected butynol can be deprotonated and used as a nucleophile for an aldehyde to comprise of the other half of the molecule.
The aldehyde component also contains a cyclohexene motif, which is a classic disconnection pattern for a Diels-Alder reaction. A Diels-Alder reaction is a pericyclic cycloaddition that could be done here with butadiene, but the reaction also requires a dienophile with and electron-withdrawing group directly attached. At first sight, we cannot the aldehyde carbonyl is too far away from the C=C alkene bond, being unconjugated. However, in a retrosynthesis, we could simply disconnect with a homologation reaction. A homologation reaction is one that increases a carbon chain length to the next member of a homologous series in organic chemistry. In this case, we need to add a methylene unit (CH2), and an aldehyde can be homologated by Wittig reaction with a specific ylid. This ylid is methoxymethylenetriphenylphosphine (Ph3P=CH(OMe)), which can be prepared by P-alkylation of triphenylphoshine, and subsequent deprotonation of the phosphonium salt to the ylid by LDA, for example. This reaction forms an enol ether product and this product can be revealed as the homologated aldehyde on further reaction with aqueous acid.
Комментарии
 0:07:29
0:07:29
 0:25:41
0:25:41
 0:11:55
0:11:55
 0:12:11
0:12:11
 0:17:41
0:17:41
 0:32:26
0:32:26
 1:05:05
1:05:05
 0:11:13
0:11:13
 0:02:47
0:02:47
 0:12:45
0:12:45
 0:46:08
0:46:08
 0:15:11
0:15:11
 0:22:36
0:22:36
 0:08:32
0:08:32
 0:00:31
0:00:31
 0:29:52
0:29:52
 0:12:06
0:12:06
 0:14:24
0:14:24
 0:04:02
0:04:02
 0:04:54
0:04:54
 0:13:54
0:13:54
 0:08:13
0:08:13
 0:11:16
0:11:16
 0:12:54
0:12:54