filmov
tv
Howard Chang (Stanford, HHMI) 2: LncRNA Function at the RNA Level: Xist

Показать описание
In this talk, Dr. Howard Chang describes epigenomic approaches pioneered by his lab and the role of long-noncoding RNAs (lncRNAs) in regulating gene expression.
In Part 1 of this series, Dr. Howard Chang introduces epigenomics, the study of DNA regulatory mechanisms that determine which genes are turned on or off in cells at specific times. The epigenome integrates signals from the environment to modify expression of the DNA blueprint inherited from an individual’s parents. Chang’s lab has pioneered techniques to map the landscape of chromatin, the complex of DNA, RNA and protein that organizes the genome and regulates gene expression. One example is ATAC, the Assay of Transposase Accessible Chromatin, which uses a bacterial transposase to mark open chromatin and identify genes that are likely turned “on”.
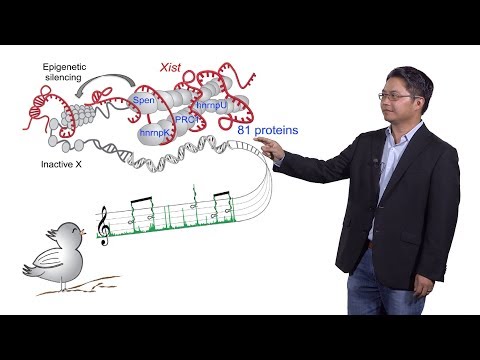
In his Part 2, Chang introduces long noncoding RNAs, or lncRNAs. As their name suggests, lncRNAs are not translated into proteins, and initially their functions were poorly understood. Chang’s group has developed technologies to better understand the function of lncRNAs. For example, his lab characterized the protein partners that interact with Xist, a canonical lncRNA that mediates X chromosome inactivation. They found that the protein Spen is necessary for X chromosome silencing. Interestingly, Spen has likely been co-opted by mammalian cells to inactivate the X chromosome via viral mimicry.
In his Part 3, Chang reminds us that every lncRNA gene has its own set of DNA regulatory elements, such as enhancers and promoters. These regulatory elements can confer functionality to lncRNA genes. Chang shares the research story of a mysterious lncRNA known as PVT1, which is frequently co-amplified with the proto-oncogene MYC in human cancers. His group found that PVT1 promoter activity is inversely correlated with MYC expression - when one is up, the other is down. Finally, Chang shows that the PVT1 and MYC promoters compete for four enhancers located within the PVT1 gene locus.
Speaker Biography:
Howard Chang is the Virginia and D. K. Ludwig Professor of Cancer Genomics and a professor of dermatology and genetics at Stanford University. He is an Investigator of the Howard Hughes Medical Institute. He studied biochemistry at Harvard University and completed a doctorate in biology at the Massachusetts Institute of Technology and medical degree at Harvard Medical School. The Chang lab pioneers new technologies for probing the function of the non-coding genome.
Комментарии
 0:24:53
0:24:53
 0:39:30
0:39:30
 0:23:55
0:23:55
 0:47:51
0:47:51
 0:34:27
0:34:27
 0:06:39
0:06:39
 0:41:58
0:41:58
 0:47:51
0:47:51
 0:01:41
0:01:41
 0:34:13
0:34:13
 0:18:24
0:18:24
 1:03:47
1:03:47
 0:29:07
0:29:07
 0:31:28
0:31:28
 1:03:47
1:03:47
 0:34:43
0:34:43
 0:02:16
0:02:16
 0:59:07
0:59:07
 0:25:11
0:25:11
 1:00:57
1:00:57
 0:02:49
0:02:49
 0:05:46
0:05:46
 0:27:12
0:27:12
 0:59:43
0:59:43