filmov
tv
R1.2.2 Hess's Law [SL IB Chemistry]

Показать описание
Determine the enthalpy change of a reaction that is the sum of two or three reactions with known enthalpy changes. You don't need to know Hess's Law (!) but you need to be able to use it three ways. The law allows you to work out delta H for reactions, given the delta H for other reactions. You can even work out delta H for reactions that cannot occur. Look up Hess's Law you lazy bum!
R1.2.2 Hess's Law [SL IB Chemistry]
R1.2.2 Hess's law example
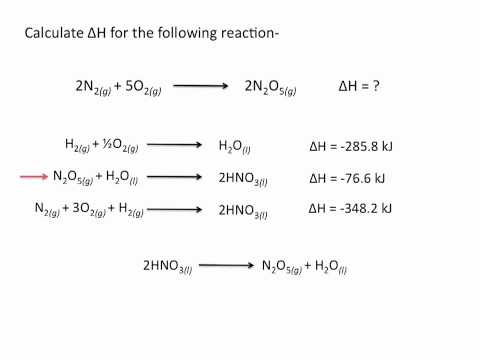
Hess's Law Problems & Enthalpy Change - Chemistry
Reactivity 1.2.2 Hess's Law [IB Chemistry SL/HL]
R1.2.2 - What is Hess's law?
Hess's Law (IB Chemistry R1.2)
IB Chemistry R1.2 Energy cycles in reactions - Hess's Law
R1.1.2 - How do we apply Hess's law to enthalpy changes of reaction?
5.2 Hess's law (SL)
IB Chemistry Topic 5.2 & 5.3: Hess's Law & Bond Enthalpies
Hess' law worked example (homework problem solution)
DSE/GCE/IB Thermochemistry - Hess's Law :Using 2 equations for the calculation of enthalpy chan...
Hess's Law - Chemistry Tutorial
IB Chemistry-Hess's Law (CHeM In 3-Episode 4)
Hess' Law (Enthalpy Changes) - IB Chemistry Revision Course
IB Chemistry: Hess Cycles
Hess's Law Practice Problem
Hess's Law
Solving Heat of Formation Problems (IB Chemistry)
5.2 Enthalpy cycles (SL)
5.3.1 Determine the enthalpy change of a reaction using Hess' law.
R1.1.2 - Which energy changes do we measure in calorimetry?
3 HL Entropy
Practicing Hess's Law: Chemistry Sample Problem
Комментарии
 0:07:05
0:07:05
 0:03:55
0:03:55
 0:14:03
0:14:03
 0:08:40
0:08:40
 0:06:32
0:06:32
 0:21:56
0:21:56
 0:19:35
0:19:35
 0:04:58
0:04:58
 0:05:04
0:05:04
 0:04:56
0:04:56
 0:04:12
0:04:12
 0:32:11
0:32:11
 0:11:23
0:11:23
 0:04:23
0:04:23
 0:12:07
0:12:07
 0:07:33
0:07:33
 0:12:11
0:12:11
 0:08:43
0:08:43
 0:11:12
0:11:12
 0:05:11
0:05:11
 0:06:51
0:06:51
 0:06:03
0:06:03
 0:08:53
0:08:53
 0:09:34
0:09:34