filmov
tv
Meso Compounds

Показать описание
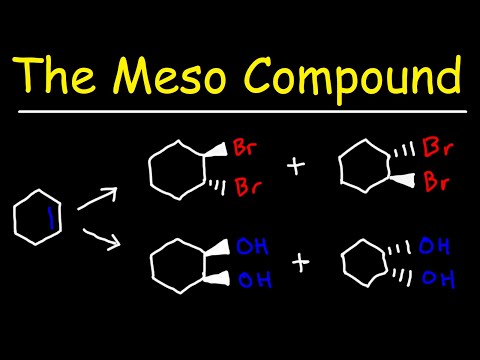
This organic chemistry video tutorial explains how to draw a meso compound and how to identify them. Meso compounds are achiral molecule with chiral centers. They have an internal plane of symmetry and are superimposable images. Meso compounds are optically inactive and they are identical molecules.
Organic Chemistry - Video Lessons:
______________________________
Finding Chirality Centers:
Chiral and Achiral Molecules:
Stereochemistry:
Enantiomers:
Diastereomers:
______________________________
Stereoisomers:
Optical Activity & Specific Rotation:
Enantiomeric Excess Test Question:
SN2 SN1 E1 E2 Reaction Mechanisms:
______________________________
Alkene Reactions Review:
Alkyne Reactions Review:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Full-Length Videos and Worksheets:
Organic Chemistry - Video Lessons:
______________________________
Finding Chirality Centers:
Chiral and Achiral Molecules:
Stereochemistry:
Enantiomers:
Diastereomers:
______________________________
Stereoisomers:
Optical Activity & Specific Rotation:
Enantiomeric Excess Test Question:
SN2 SN1 E1 E2 Reaction Mechanisms:
______________________________
Alkene Reactions Review:
Alkyne Reactions Review:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Full-Length Videos and Worksheets:
Meso Compounds
Stereochemistry: Meso Compounds, Diastereomers
Enantiomers, Diastereomers and Meso Compounds: Multiple Chiral Centers
Meso compounds | Stereochemistry | Organic chemistry | Khan Academy
Identifying Meso Compounds
What is a Meso Compound?
Meso Compounds
Meso Compounds
1,3-Oxathiolium-4-olates/ 1,3-Oxathiolium-5-olates/ Meso ionic Compounds
What’s a Meso Compound?
5.3 Molecules with Multiple Chiral Centers | Enantiomers, Diastereomers, and Meso Compounds | OChem
Meso Compounds and Chiral Resolution | Organic Chemistry Lessons
The 3 rules of meso compounds
Enantiomers, Diastereomers and Meso Compounds #neet2023 #jeeadvanced #organicchemistry
Meso Compounds, Enantiomers, and Alkene Addition Reactions
Introduction to Meso Compounds for Organic Chemistry
🔥Super Trick to Learn Concept of Meso Compounds in 30 sec #chemistry #organic #jee #neet
Meso Compounds - Stereochemistry | Organic Chemistry
Meso compounds
Stereoisomers: Enantiomers, Diastereomers, and Meso Compounds!
Meso Compound
Meso compounds Trick | IIT JEE & NEET | Vineet Khatri Sir | ATP STAR Kota
What are meso compounds? Why they are optically inactive? (Haloalkane and haloarenes)
Dr Ahmed Mutanabbi Abdula Stereochemistry. Meso compounds
Комментарии
 0:07:55
0:07:55
 0:07:44
0:07:44
 0:09:05
0:09:05
 0:09:48
0:09:48
 0:00:59
0:00:59
 0:03:10
0:03:10
 0:04:08
0:04:08
 0:18:33
0:18:33
 0:10:25
0:10:25
 0:13:37
0:13:37
 0:24:30
0:24:30
 0:09:10
0:09:10
 0:04:11
0:04:11
 0:01:00
0:01:00
 0:08:37
0:08:37
 0:07:25
0:07:25
 0:00:37
0:00:37
 0:04:27
0:04:27
 0:02:11
0:02:11
 0:17:37
0:17:37
 0:05:54
0:05:54
 0:03:57
0:03:57
 0:04:24
0:04:24
 0:09:56
0:09:56