filmov
tv
GST Pull Down Assay | Protein-Protein Interaction Using Pull Down Assay |

Показать описание
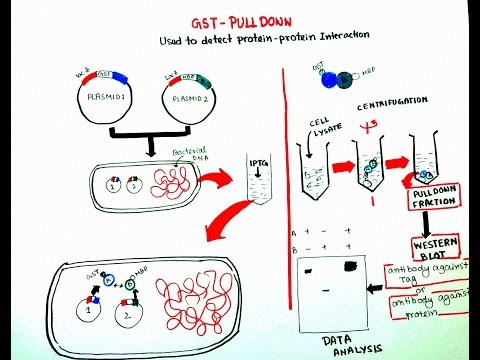
The glutathione S-transferase (GST) pull-down assay is a relatively easy, straightforward method to identify potential binding partners.
The method is also extensively used to confirm known interactions and to map interaction sites.
The pull-down method relies on the immobilization of a GST fusion protein on glutathione sepharose beads that serve as a solid phase.
The first step requires the expression of the protein of interest as a fusion protein with the GST moiety.
After binding of the GST fusion protein to the glutathione sepharose matrix, the mixture is incubated with whole-cell homogenate or a purified protein or more specifically, the Target proteins tgat are usually lysates of cells either labeled with [35S]methionine or unlabeled, depending on the method used to assay the interaction between the target and the probe.
Nonbound material is washed off the column, and subsequently the binding complex is eluted.
Upon elution, the mixture is resolved by (SDS-PAGE) and analyzed by Coomassie staining, silver staining, or Western blot....
The method is also extensively used to confirm known interactions and to map interaction sites.
The pull-down method relies on the immobilization of a GST fusion protein on glutathione sepharose beads that serve as a solid phase.
The first step requires the expression of the protein of interest as a fusion protein with the GST moiety.
After binding of the GST fusion protein to the glutathione sepharose matrix, the mixture is incubated with whole-cell homogenate or a purified protein or more specifically, the Target proteins tgat are usually lysates of cells either labeled with [35S]methionine or unlabeled, depending on the method used to assay the interaction between the target and the probe.
Nonbound material is washed off the column, and subsequently the binding complex is eluted.
Upon elution, the mixture is resolved by (SDS-PAGE) and analyzed by Coomassie staining, silver staining, or Western blot....
GST pulldown | GST Pull Down Assay | Protein-Protein Interaction Using Pull Down Assay
GST Pull Down Assay | Protein-Protein Interaction Using Pull Down Assay |
Pull down assay for protein-protein interaction | Pull Down Assay Protocol | Biology Lectures |
Pull-Down Assay Protocol
GST Pull Down Assay & Co Immunoprecipitation | Protein-Protein Interactions | PENS#92
In situ pulldown assays: a new approach to ligand discovery
Pull-down assays (co-IPs (co-immunoprecipitations), etc) - what, how, & what to look for
Pull-Down Assay, introduzione alla pesca proteica
An Introduction to Pull Down Assay
Pull-down assay (protein-protein interaction)
techniques to study protein protein interaction
Interpretting co-IP & other pulldown figures
Co Immunoprecipitation | Immmunorecipitation | Co IP Assay Principle | Procedure | Technique |
Pull Down Assay | Protein-Protein Intraction Using Pull Down Assay |
Biotin-based Pulldown Assay to Validate mRNA Targets | Protocol Preview
Pull-Down Assay
RNA Targets Identification by RNA Pull-down Procedure | Protocol Preview
Chromatography (Part 2 of 2): GST Tagging
protein interaction technoloiges Pull Down Assays
Yeast two hybrid system | Protein - protein interaction
Pull Down Assay
Immunoprecipitation with an Affinity Gel Protocol
Pull down assay
GST Fusion Protein Gel
Комментарии
 0:02:44
0:02:44
 0:01:39
0:01:39
 0:03:09
0:03:09
 0:11:58
0:11:58
 0:15:13
0:15:13
 0:03:02
0:03:02
 0:45:31
0:45:31
 0:02:09
0:02:09
 0:07:10
0:07:10
 0:03:07
0:03:07
 0:09:22
0:09:22
 0:08:53
0:08:53
 0:08:07
0:08:07
 0:02:34
0:02:34
 0:02:01
0:02:01
 0:03:25
0:03:25
 0:02:01
0:02:01
 0:05:30
0:05:30
 0:33:27
0:33:27
 0:03:09
0:03:09
 0:03:02
0:03:02
 0:03:08
0:03:08
 0:01:54
0:01:54
 0:00:25
0:00:25