filmov
tv
Electrolysis of copper sulphate (CuSO4) experiment|#shorts #electrolysisexperiment #electrochemistry

Показать описание
#electrochemistry #electrolysis #shorts #shortvideo #experiment #scienceexperiment #class12th #electrolysisexperiment #iitjee #jeemains #neet
Electrolysis of copper sulfate CuSO4 (aq) using graphite and copper electrodes - GCSE Chemistry
Electrolysis of Copper Sulfate using Copper Electrodes
Electrolysis of copper sulphate (CuSO4) experiment|#shorts #electrolysisexperiment #electrochemistry
electrolysis of copper sulphate solution using copper electrodes ll electroplating using copper
ELECTROLYSIS OF COPPER SULPHATE (CuSO4)
Pearson Edexcel (9-1) Comb. Sciences - electrolysis of copper sulfate with copper electrodes
Electrolysis of Copper sulphate #shorts #science #ytshorts #diy
Electrolysis of Copper Sulfate in RamZland!⚗️ (CuSO4)
Electrolysis of Copper Sulphate(CuSO4)|Chemistry Practicals
Purifying Copper | Reactions | Chemistry | FuseSchool
Electrolysis copper extraction
Pearson Edexcel (9-1) | SHORTS | Electrolysis of copper sulfate | GCSE Combined Sci | GCSE Chemistry
CHEMISTRY: Electrolysis of Copper Sulfate Solution with Inert Electrodes and Copper Electrodes
Preparation of Sulphuric Acid (H2SO4) With Electrolysis of Copper Sulphate (CuSO4)
CuSO4 Electrolysis - Making Sulfuric Acid (revisited)
Electrolysis of Copper Sulfate using Inert Electrodes
Electrolysis of copper sulphate @dkstudentoo
Electrolysis of Copper Sulphate Solution
Electrolysis of Copper Sulphate (CuSO4) Experiment | Electrolysis Class 12 | #scienceexperiment
Electrolysis of Copper Sulphate
ICSE Std-10 Chemistry- Reasons of Electrolysis of Copper Sulphate CuSO4 || Electrolysis
Electroplating of copper | Diy Electroplating #shorts #science #ytshorts #diy
Electrolysis of aqueous copper sulfate
Electrolysis Of Copper(ii) Sulphate Using Copper Electrodes
Комментарии
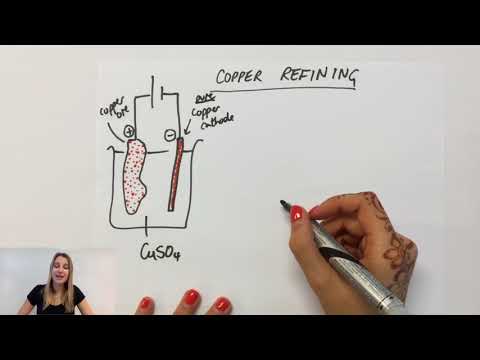 0:05:11
0:05:11
 0:01:13
0:01:13
 0:01:00
0:01:00
 0:01:40
0:01:40
 0:08:39
0:08:39
 0:12:29
0:12:29
 0:01:00
0:01:00
 0:01:52
0:01:52
 0:04:19
0:04:19
 0:04:32
0:04:32
 0:00:17
0:00:17
 0:02:38
0:02:38
 0:05:41
0:05:41
 0:04:48
0:04:48
 0:01:38
0:01:38
 0:01:35
0:01:35
 0:01:48
0:01:48
 0:04:46
0:04:46
 0:13:05
0:13:05
 0:01:47
0:01:47
 0:08:51
0:08:51
 0:01:00
0:01:00
 0:03:00
0:03:00
 0:09:49
0:09:49