filmov
tv
How to Calculate Effective Nuclear Charge - Slater's Rules - Chemistry

Показать описание
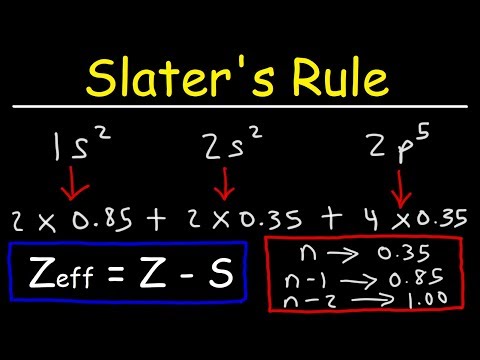
In this video, the concept of effective nuclear charge is discussed in detail. A definition of effective nuclear charge is provided alongside an explanation of why it arises. The neon atom is analyzed in this particular problem. The electron configuration of the neon atom is written out and a picture of the atom is then drawn according to the Bohr model. The effective nuclear charge felt by a valence electron and a core electron is then calculated using Slater's Rules and the equation Zeff = Z - S. Slater's Rules are listed out and the concept and consequences of electron shielding/screening are discussed at length.
Concepts discussed in this video include:
1. Effective nuclear charge
2. Nuclear charge
3. Slater's rules
4. Electron screening
5. Energy levels
6. Bohr model
7. Electron configurations
8. The Periodic Table
9. Drawing an atom with discrete energy levels
Concepts discussed in this video include:
1. Effective nuclear charge
2. Nuclear charge
3. Slater's rules
4. Electron screening
5. Energy levels
6. Bohr model
7. Electron configurations
8. The Periodic Table
9. Drawing an atom with discrete energy levels
 0:07:14
0:07:14
 0:12:30
0:12:30
 0:04:47
0:04:47
 0:17:27
0:17:27
 0:09:31
0:09:31
 0:04:05
0:04:05
 0:01:47
0:01:47
 0:12:56
0:12:56
 0:02:58
0:02:58
 0:05:25
0:05:25
 0:07:17
0:07:17
 0:15:17
0:15:17
 0:03:05
0:03:05
 0:10:50
0:10:50
 0:00:58
0:00:58
 0:02:12
0:02:12
 0:08:05
0:08:05
 0:16:39
0:16:39
 0:17:32
0:17:32
 0:08:10
0:08:10
 0:07:20
0:07:20
 0:03:26
0:03:26
 0:05:15
0:05:15
 0:07:46
0:07:46