filmov
tv
Percent Composition By Mass
Показать описание
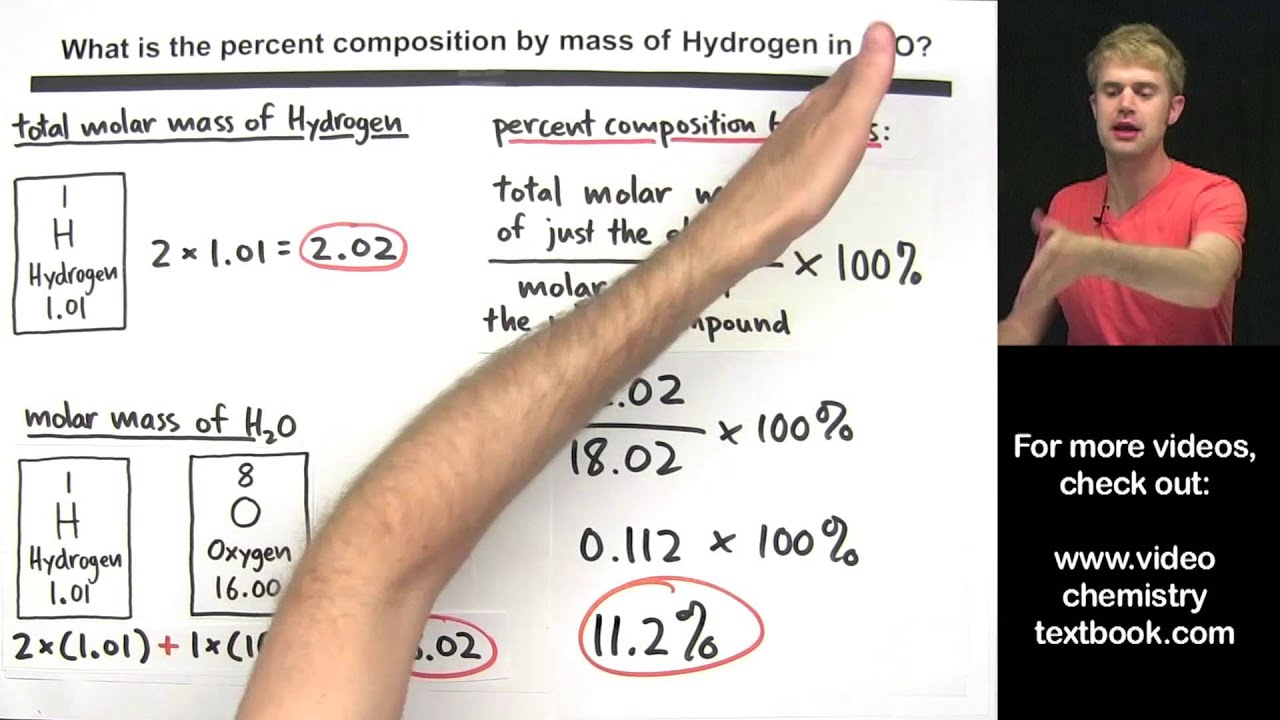
We'll learn how to calculate percent composition by mass, and we'll work through a number of practice problems. To calculate percent by mass, you need to determine two things: the mass of just the element, and the molar mass of the whole compound. Then, you take the molar mass of just the element and divide it by the molar mass of the whole compound, and multiply by 100%.
Percent Composition By Mass
Percent Composition By Mass
How to Find the Percent Composition by Mass for a Compound
Percent Composition By Mass Part 1
How to Find the Percent Composition by Mass for H2O (Water)
How to Find the Percent Composition by Mass for C2H6 (Ethane)
Empirical Formula & Molecular Formula Determination From Percent Composition
Percent Composition from Mass Data
How To calculate Percentage Mass | Chemical Calculations | Chemistry | FuseSchool
Percent Composition Problem Given Mass (Grams)
Calculating Percent Composition by Mass
1.2 Calculating percentage composition by mass
Percent Composition Common Mistakes
Quick Revision - Percentage Composition by Mass
How to Find the Percent Composition by Mass for CO2 (Carbon dioxide)
How to Find the Percent Composition by Mass for C2H5OH (Ethanol)
Percent Composition by Mass
How to Find the Percent Composition by Mass for FeCl3 . 6H2O
How to Find the Percent Composition by Mass for Na2CO3 (Sodium carbonate)
Percent Composition by Mass for C2H4O2 (Acetic acid)
How to Find the Percent Composition by Mass for CH4 (Methane)
How to Find the Percent Composition by Mass for H2SO4 (Sulfuric acid)
How to Find the Percent Composition by Mass for Sodium sulfate (Na2SO4)
3.5 Percent Composition
Комментарии
 0:17:01
0:17:01
 0:06:58
0:06:58
 0:05:59
0:05:59
 0:02:20
0:02:20
 0:01:46
0:01:46
 0:01:49
0:01:49
 0:11:00
0:11:00
 0:02:40
0:02:40
 0:03:27
0:03:27
 0:05:01
0:05:01
 0:10:05
0:10:05
 0:01:43
0:01:43
 0:02:47
0:02:47
 0:01:46
0:01:46
 0:01:54
0:01:54
 0:02:45
0:02:45
 0:02:05
0:02:05
 0:01:33
0:01:33
 0:02:23
0:02:23
 0:02:43
0:02:43
 0:01:48
0:01:48
 0:02:21
0:02:21
 0:01:59
0:01:59
 0:14:40
0:14:40