filmov
tv
formation & reaction of amide enolates

Показать описание
Amides are weakly acidic at the alpha-carbon with a pKa of around 30. LDA is an adequate base for the formation of amide enolates through deprotonation. Note that only tertiary amides in which the amide nitrogen has two R-groups can be deprotonated to make an enolate. The resulting enolate is nucleophilic and can attack carbonyl compounds, like aldehydes. The resulting beta-hydroxy amide and be further reacted with base to generate an alpha,beta-unsaturated amide. Amide enolates can also undergo reaction with alkyl halides through an SN2 reaction to give an alkylated amide.
 0:11:17
0:11:17
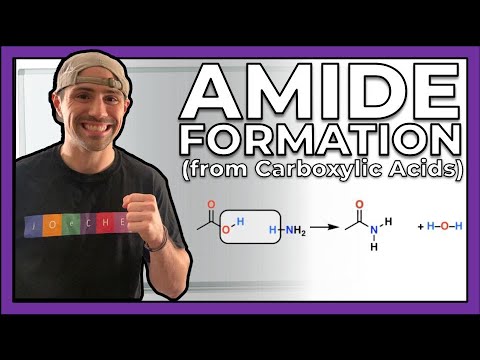 0:05:45
0:05:45
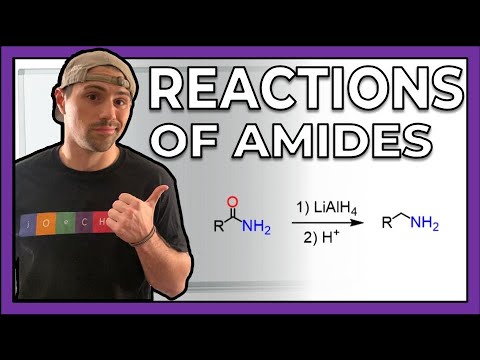 0:06:21
0:06:21
 0:03:08
0:03:08
 0:09:01
0:09:01
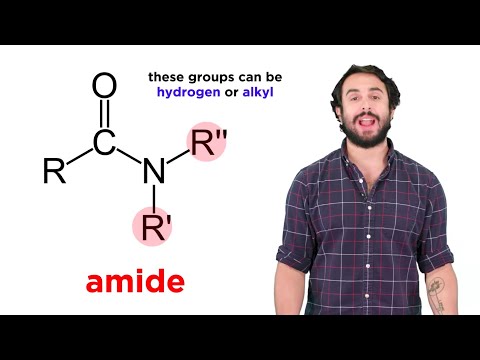 0:07:52
0:07:52
 0:04:44
0:04:44
 0:03:19
0:03:19
 0:24:10
0:24:10
 0:02:16
0:02:16
 0:11:54
0:11:54
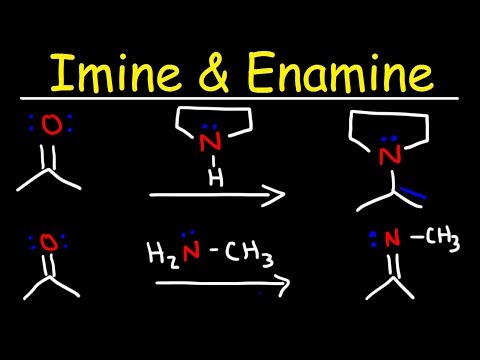 0:12:36
0:12:36
 0:00:47
0:00:47
 0:08:00
0:08:00
 0:05:13
0:05:13
 0:28:00
0:28:00
 0:11:25
0:11:25
 0:04:51
0:04:51
 0:04:09
0:04:09
 0:05:36
0:05:36
 0:01:53
0:01:53
 0:03:23
0:03:23
 0:00:15
0:00:15
 0:04:02
0:04:02