filmov
tv
9.2 Voltaic cells (SL)
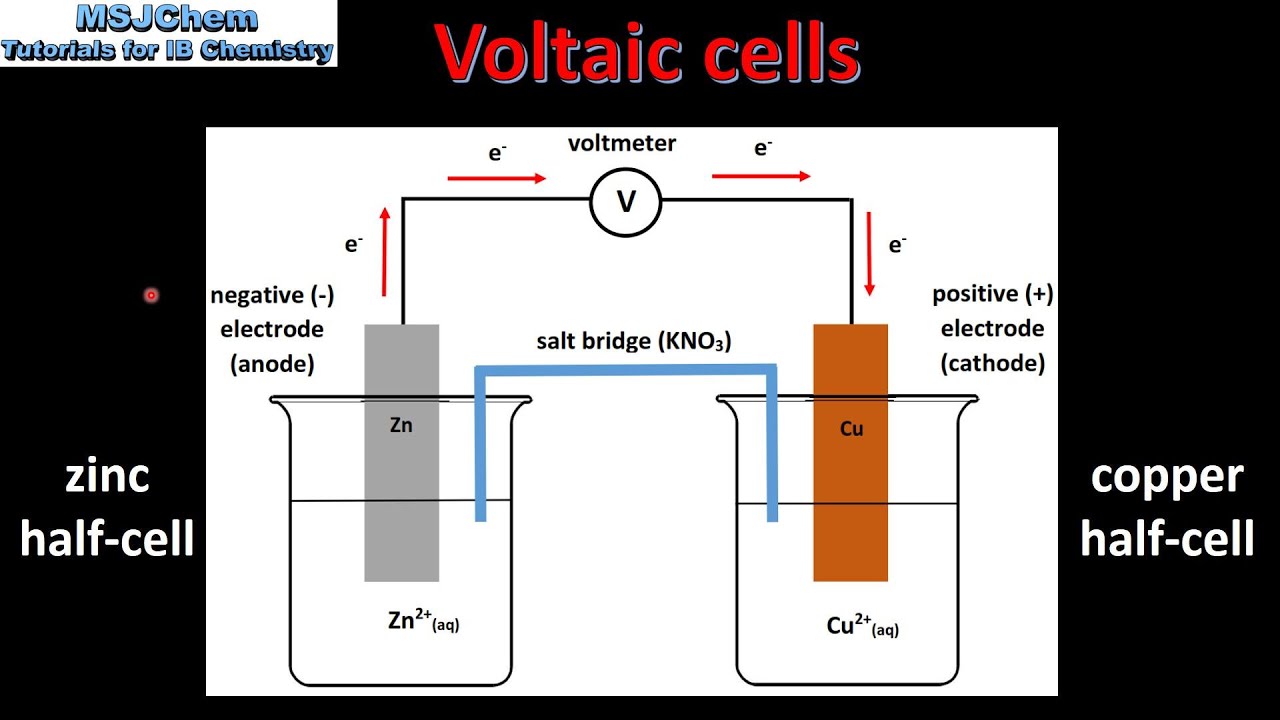
Показать описание
9.2 Voltaic cells
Understandings:
Oxidation occurs at the anode (negative electrode) and reduction occurs at the cathode (positive electrode) in a voltaic cell.
Applications and skills:
Construction and annotation of both types of electrochemical cells.
Explanation of how a redox reaction is used to produce electricity in a voltaic cell.
Distinction between electron and ion flow in voltaic cells.
Link to worksheet:
Understandings:
Oxidation occurs at the anode (negative electrode) and reduction occurs at the cathode (positive electrode) in a voltaic cell.
Applications and skills:
Construction and annotation of both types of electrochemical cells.
Explanation of how a redox reaction is used to produce electricity in a voltaic cell.
Distinction between electron and ion flow in voltaic cells.
Link to worksheet:
Introduction to Galvanic Cells & Voltaic Cells
IB Chemistry Topic 9 Redox processes Topic 9.2 Electrochemical cells SL
Discussion 9-2 - Electrochemical Cells SL ONLY
R3.2.6 Voltaic cells
IB Chemistry Topic 9.2: Electrochemical cells
R3.2.5/6 Explain how a redox reaction is used in a voltaic cell [SL IB Chemistry]
Experiment #9 Electrochemical Cells
IB Chemistry Topic 9.2 PART 7: Construct Electrolytic Cells & How it is Different from Voltaic C...
1.2.2- What makes lithium-ion cells different from electrochemical cells.
IB Chemistry Topic 9.2 & 19.1 PART 6: Calculate Voltaic Cell Electrode Potential & Spontanei...
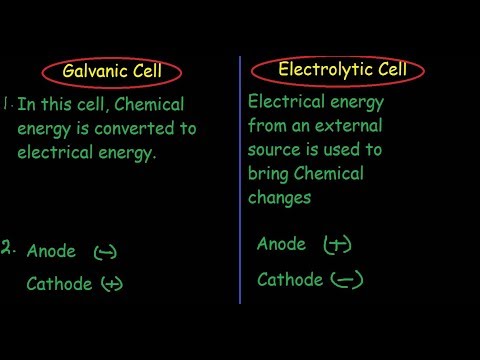
Galvanic Cell Vs Electrolytic Cell differences
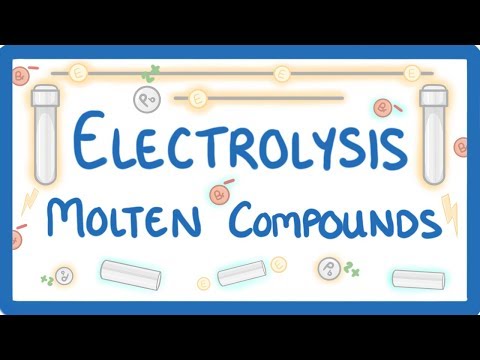
GCSE Chemistry - Electrolysis Part 1 - Basics and Molten Compounds #40
Cellular Respiration (UPDATED)
Oxidation and Reduction Reactions - Basic Introduction
IB Chemistry Topic 9.2 PART 5 Oxidation Reduction: 5 Steps to Constructing Voltaic (Galvanic) Cells
GCSE Chemistry - Fuel Cells #45
Galvanic (voltaic) cells | Applications of thermodynamics | AP Chemistry | Khan Academy
Breaking down electrolytic cells #electrochemistry
Lecture 4.9 -solving a voltaic cell question
Galvanic Cells Explained
Electrolysis of Water - Electrochemistry
Experiment #8: Electrochemistry - Voltaic Cells - Prelab Discussion
IB Chemistry Topic 9 Redox processes Topic 19.1 Electrochemical cells HL
Contrasting Voltaic and Electrolytic Cells
Комментарии
 0:27:42
0:27:42
 0:07:21
0:07:21
 0:21:41
0:21:41
 0:06:00
0:06:00
 0:06:41
0:06:41
 0:09:27
0:09:27
 1:04:30
1:04:30
 0:12:29
0:12:29
 0:18:11
0:18:11
 0:15:00
0:15:00
 0:02:33
0:02:33
 0:04:07
0:04:07
 0:08:47
0:08:47
 0:16:05
0:16:05
 0:14:36
0:14:36
 0:07:27
0:07:27
 0:09:12
0:09:12
 0:00:51
0:00:51
 0:21:22
0:21:22
 0:03:15
0:03:15
 0:13:12
0:13:12
 0:13:18
0:13:18
 0:16:28
0:16:28
 0:17:41
0:17:41