filmov
tv
Determining Chirality Using Planes of Symmetry

Показать описание
Practice identifying planes of symmetry in achiral molecules and the lack thereof in chiral molecules.
Determining Chirality Using Planes of Symmetry
identifying meso and chirality in compounds with planes of symmetry
Finding Chirality Centers
How to Find Chiral Centers & the Difference Between Chiral vs Achiral Molecules | Organic Chemis...
Chiral and Achiral Molecules - Allenes and Alkenes
Achiral carbon and Chiral carbon atoms #chirality #shorts
Chirality|Basic Concept Explained
chirality, meso compounds and planes of symmetry
58: Symmetry and chirality
07.03 Determining Whether a Molecule is Chiral
Determining (A)chirality
What is a Chiral Center? #organicchemistry
Optical rotation of sugars – chirality
Determining Chirality by Generating the Mirror Image
Determine R and S configuration using your hands
Chiral Molecule Question #chemistry #organicchemistry #shorts
Chirality and Stereochemistry Practice Problems
Optical Activity - Specific Rotation & Enantiomeric Excess - Stereochemistry Youtube
Lecture Video Ch5 8 Determine Chiral Centers
Identifying chiral or achiral and chiral centers - chiral, achiral, chrial center- organic chemistry
# Find plane of symmetry Chiral/achiral molecules @ Veena Dixit Chemistry IIT jee
Chirality: Tips and Tricks to Finding Chiral Carbons for Organic Chemistry
Identifying chiral carbon atoms example
Determine Chirality And Drawing Chiral Molecules
Комментарии
 0:14:37
0:14:37
 0:06:39
0:06:39
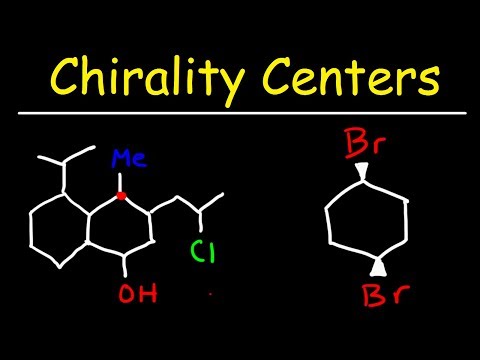 0:06:03
0:06:03
 0:03:40
0:03:40
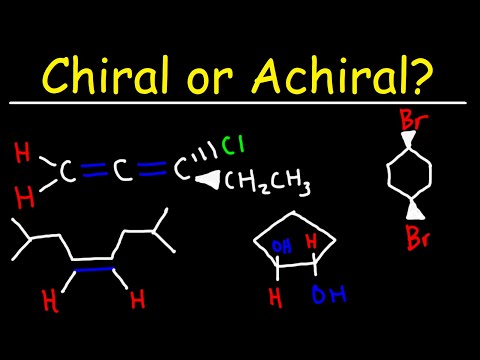 0:05:17
0:05:17
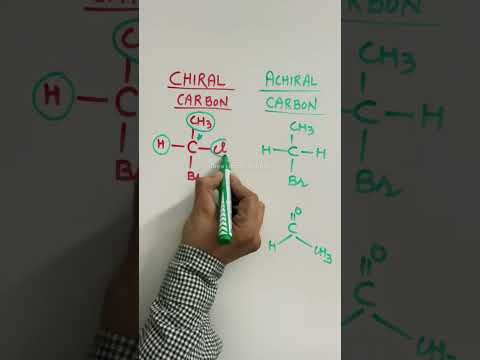 0:00:35
0:00:35
 0:03:10
0:03:10
 0:06:33
0:06:33
 0:04:36
0:04:36
 0:12:13
0:12:13
 0:07:37
0:07:37
 0:00:08
0:00:08
 0:04:02
0:04:02
 0:09:35
0:09:35
 0:05:25
0:05:25
 0:00:19
0:00:19
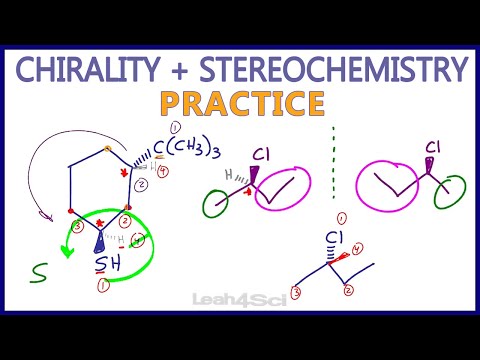 0:12:50
0:12:50
 0:22:47
0:22:47
 0:05:57
0:05:57
 0:11:50
0:11:50
 0:01:00
0:01:00
 0:22:54
0:22:54
 0:02:44
0:02:44
 0:25:01
0:25:01