filmov
tv
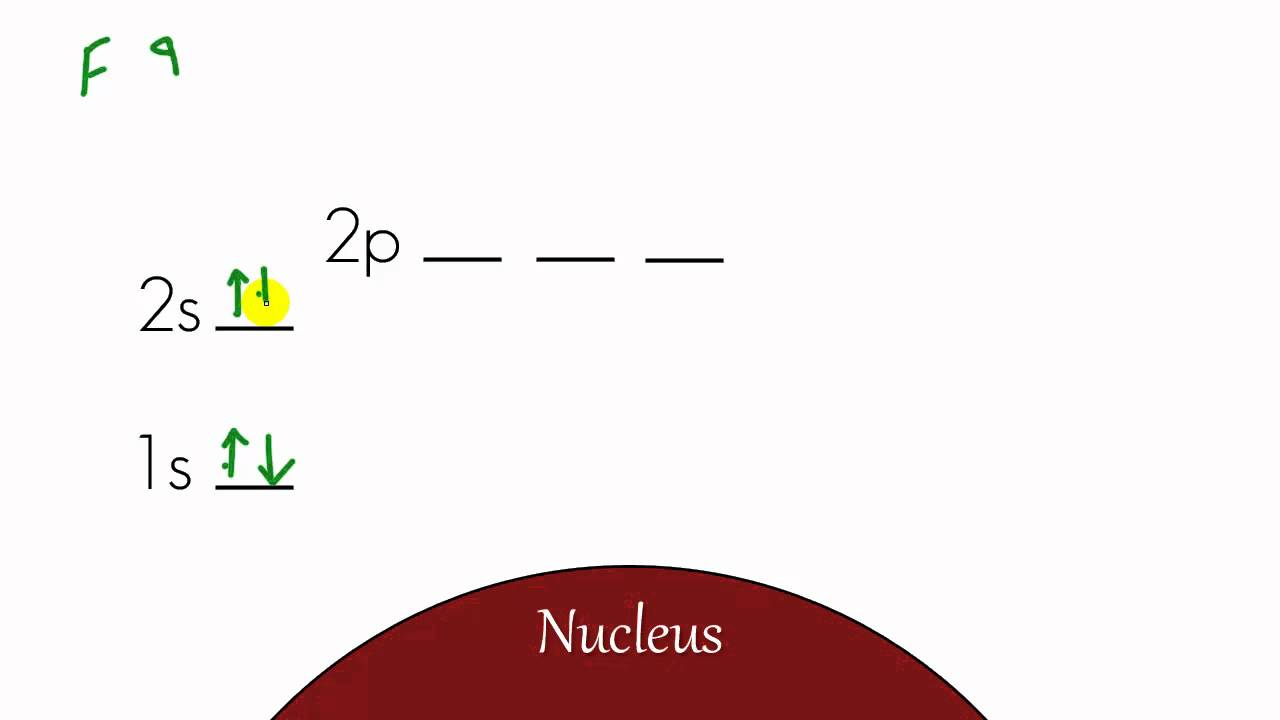
Chemistry Lesson - 12 - Energy Level Diagram and Electron Configuration
Показать описание
12th Chemistry | Last 1.5day=65+/70 | Top 10 questions in each units-half yearly exam 2024
Plus Two Christmas Exam Chemistry - Super 60 | Xylem Plus Two
12th Chemistry | 70/70 Confirm | last minute 2m 3m 5m-Half yearly exam 2024
How to Study Organic Chemistry for Class 12 Boards 2025
12th chemistry 1 day = 65+/70 important 2,3,5m | 12th chemistry half yearly important questions 2024
class 12 chemistry chapter 12 full explanation/aldehyde ketone and carboxylic acid class 12 one shot
how to study chemistry class 12 | important chapters for class 12 chemistry #shorts #short
Exam Capsule : Chemistry class 12 chapter 5 subjective and Objective bihar board
12th chemistry confirm 70/70 marks - 12th chemistry half yearly important questions 2024
24 HOURS=98+ MARKS 🔥|| PREBOARD STRATEGY FOR CLASS 12 CHEMISTRY 2024-2025 || MUNIL SIR
Alcohol Phenol and Ethers Class 12 Chemistry | NCERT | Organic |CBSE NEET JEE
class 12 | chemistry | aldehyde, ketone and carboxylic acid | all name reactions | CBSE | JEE | NEET
Conversion Reactions in Organic Chemistry | Important Conversions | Class 12
Solutions Chemistry Class 12 | Chapter 2 | Introduction | JEE 2024 | EAMCET 2024 | Jummidi sir
Alkanes | Homologous series | General Organic Chemistry #chemistry #Hydrocarbons #organicchemistry
GENERAL CHEMISTRY explained in 19 Minutes
Vijeta 2025 | Haloalkanes and Haloarenes One Shot | Chemistry | Class 12th Boards
Class 12th Chemistry Official Model Paper | 12th Chemistry Model Paper 2025 | UP Board Exams 2025
PREBOARDS 🔥 Most Important Topics of Chemistry for Pre Boards | Class 12 Chemistry
Organic Chemistry Name Reactions | Class 12 Boards 2025 | Most Important Name reactions |
Class 12th Chemistry Chapter 11 & 12 Complete Revision | Bihar Board 12th Chemistry Exam 2025
Organic Chemistry Class 11 | Chapter 12 NCERT CBSE NEET JEE #1
Solid State Class 12 Chemistry| Chapter 1 One Shot| CBSE NEET JEE
Biomolecules Class 12 Chemistry | NCERT Chapter 14 | One Shot |CBSE NEET JEE
Комментарии
 0:11:22
0:11:22
 4:20:16
4:20:16
 0:07:41
0:07:41
 0:13:08
0:13:08
 0:12:08
0:12:08
 8:01:40
8:01:40
 0:00:51
0:00:51
 0:43:10
0:43:10
 0:13:08
0:13:08
 0:09:29
0:09:29
 1:34:39
1:34:39
 0:00:12
0:00:12
 0:16:53
0:16:53
 0:44:56
0:44:56
 0:00:16
0:00:16
 0:18:49
0:18:49
 5:16:05
5:16:05
 1:39:45
1:39:45
 0:09:56
0:09:56
 1:31:15
1:31:15
 3:46:23
3:46:23
 1:05:56
1:05:56
 2:16:39
2:16:39
 1:35:48
1:35:48