filmov
tv
Degree of Unsaturation from Molecular Structure

Показать описание
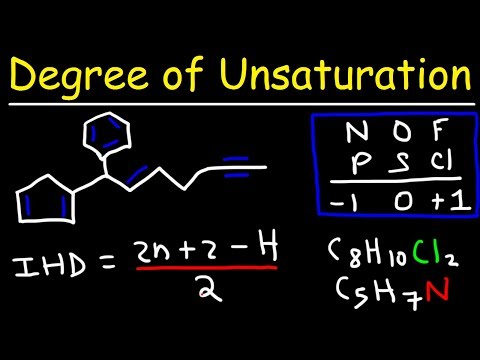
If you know the molecular structure of a substance, you can calculate its degree of unsaturation.
Each double bond contributes 1
Each triple bond contributes 2
Each RING contributes 1
Add up all those contributions, and you have you Degree of Unsaturation.
Each double bond contributes 1
Each triple bond contributes 2
Each RING contributes 1
Add up all those contributions, and you have you Degree of Unsaturation.
Degree of Unsaturation from Chemical Formula (Five Examples)
Degree of Unsaturation and Index of Hydrogen Deficiency
Degree of Unsaturation from Molecular Structure
Degrees of Unsaturation #chemistry #science #organicchem #organicchemistry #organicchemistrytutor
How to Calculate Degrees of Unsaturation Ft. Professor Dave
Calculate Degrees of Unsaturation From Molecular Formula 017
Calculating the degree of unsaturation from a molecular formula
2423 EP03 004G - Degrees of Unsaturation 001
VVI questions of solid state for +2 chemistry with concepts and PYQs 2025 #chemistry #education
2423 EP07 001B - Calculate Degrees of Unsaturation From Molecular Formula 001
' Degree Of Unsaturation ' With QuickShot Chemistry | #Deepika Ma'am | #shorts#neet#o...
How to solve molecular structure using Degrees of Unsaturation NMR/IR
Degree of Unsaturation aka Index of Hydrogen Deficiency
Calculate Degrees of Unsaturation From Molecular Structure 002
What is the degree of unsaturation in a compound with molecular formula `C_(9)H_(6)N_(4)`?
Determining the Degree of Unsaturation (Index of H Deficiency) in Hydrocarbons
Calculating Degree of Unsaturation
How many degrees of unsaturation? #organicchemistry
degree of unsaturation from a bond line structure
Degrees Of Unsaturation Chemistry Question #chemistry #shorts
10.2 Degrees of Unsaturation
Degree of Unsaturation
Calculate Units of Unsaturation From Molecular Formula 016
DEGREE OF UNSATURATION QUESTION
Комментарии
 0:04:18
0:04:18
 0:13:05
0:13:05
 0:03:18
0:03:18
 0:00:42
0:00:42
 0:03:54
0:03:54
 0:02:19
0:02:19
 0:05:40
0:05:40
 0:03:46
0:03:46
 0:10:40
0:10:40
 0:03:37
0:03:37
 0:00:12
0:00:12
 0:08:11
0:08:11
 0:16:11
0:16:11
 0:01:30
0:01:30
 0:03:02
0:03:02
 0:08:21
0:08:21
 0:04:23
0:04:23
 0:00:28
0:00:28
 0:02:57
0:02:57
 0:00:20
0:00:20
 0:05:14
0:05:14
 0:13:40
0:13:40
 0:03:18
0:03:18
 0:00:16
0:00:16