filmov
tv
Oxygen Atom 16mass number video explanation

Показать описание
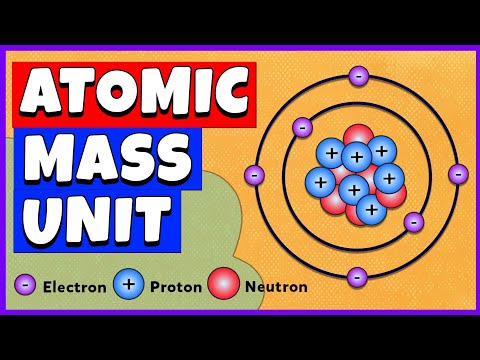
Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom.
Consider oxygen, which has an atomic number (Z) of 8. This means that oxygen atoms have 8 protons. Most atoms of oxygen also have 8 neutrons. Oxygen atoms with 8 neutrons would have a mass number of 16 (8 p+ + 8 n0), meaning they would have a mass of about 16 amu
The mass number also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.
***License for use: Free for Students for only personal use. Attribution is required in all other cases . Contact us for any legal use***
Copyright material belongs to asklearngrow
#asklearngrow #alg #asklearngrowapp #education #radiation #radioactive #alpha #beta #gamma
Consider oxygen, which has an atomic number (Z) of 8. This means that oxygen atoms have 8 protons. Most atoms of oxygen also have 8 neutrons. Oxygen atoms with 8 neutrons would have a mass number of 16 (8 p+ + 8 n0), meaning they would have a mass of about 16 amu
The mass number also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.
***License for use: Free for Students for only personal use. Attribution is required in all other cases . Contact us for any legal use***
Copyright material belongs to asklearngrow
#asklearngrow #alg #asklearngrowapp #education #radiation #radioactive #alpha #beta #gamma
 0:00:13
0:00:13
 0:03:03
0:03:03
 0:01:51
0:01:51
 0:00:17
0:00:17
 0:00:32
0:00:32
 0:02:31
0:02:31
 0:00:53
0:00:53
 0:04:16
0:04:16
 0:05:40
0:05:40
 0:44:25
0:44:25
 0:14:30
0:14:30
 0:03:42
0:03:42
 0:05:33
0:05:33
 0:11:53
0:11:53
 0:28:35
0:28:35
 0:04:13
0:04:13
 0:04:00
0:04:00
 0:05:56
0:05:56
 0:05:27
0:05:27
 0:03:56
0:03:56
 0:18:06
0:18:06
 0:52:35
0:52:35
 0:00:33
0:00:33
 0:00:16
0:00:16