filmov
tv
Converting Kc to Kp

Показать описание
Equilibrium constants can be expressed as a partial pressure of reactants and products (Kp).
Concentration and partial pressure can be related using the ideal gas law PV = nRT
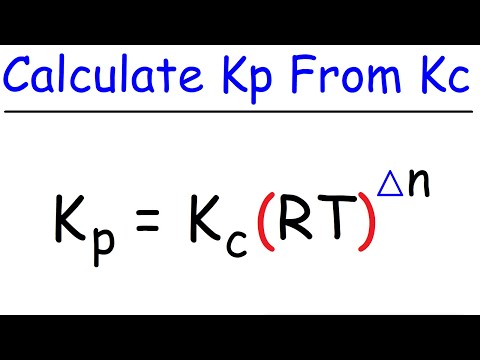
KP = KC(RT)n gas
R = 0.08205 L atm/mol K
T = Temp in K
n gas = Stoic. Coefficients of the gaseous products - Stoic. Coefficients of the gaseous reactant.
Here is an index of the entire Video Textbook of Chemistry:
Concentration and partial pressure can be related using the ideal gas law PV = nRT
KP = KC(RT)n gas
R = 0.08205 L atm/mol K
T = Temp in K
n gas = Stoic. Coefficients of the gaseous products - Stoic. Coefficients of the gaseous reactant.
Here is an index of the entire Video Textbook of Chemistry:
 0:02:54
0:02:54
 0:04:19
0:04:19
 0:03:57
0:03:57
 0:10:51
0:10:51
 0:03:20
0:03:20
 0:04:03
0:04:03
 0:07:39
0:07:39
 0:02:43
0:02:43
 0:04:42
0:04:42
 0:02:55
0:02:55
 0:08:44
0:08:44
 0:06:39
0:06:39
 0:03:03
0:03:03
 0:01:22
0:01:22
 0:05:46
0:05:46
 0:09:15
0:09:15
 0:09:33
0:09:33
 0:03:15
0:03:15
 0:10:22
0:10:22
 0:03:48
0:03:48
 0:09:13
0:09:13
 0:03:21
0:03:21
 0:53:22
0:53:22
 0:06:40
0:06:40